Clinical Evaluation of Roche SD Biosensor Rapid Antigen Test for SARS-CoV-2 in Municipal Health Service Testing Site, the Netherlands
- PMID: 33724916
- PMCID: PMC8084500
- DOI: 10.3201/eid2705.204688
Clinical Evaluation of Roche SD Biosensor Rapid Antigen Test for SARS-CoV-2 in Municipal Health Service Testing Site, the Netherlands
Abstract
Rapid detection of infection is essential for stopping the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The Roche SD Biosensor rapid antigen test for SARS-CoV-2 was evaluated in a nonhospitalized symptomatic population. We rapid-tested a sample onsite and compared results with those from reverse transcription PCR and virus culture. We analyzed date of onset and symptoms using data from a clinical questionnaire. Overall test sensitivity was 84.9% (95% CI 79.1-89.4) and specificity was 99.5% (95% CI 98.7-99.8). Sensitivity increased to 95.8% (95% CI 90.5-98.2) for persons who sought care within 7 days of symptom onset. Test band intensity and time to result correlated strongly with viral load; thus, strong positive results could be read before the recommended time. Approximately 98% of all viable specimens with cycle threshold <30 were detected. Rapid antigen tests can detect symptomatic SARS-CoV-2 infections in the early phase of disease, thereby identifying the most infectious persons.
Keywords: COVID-19; Roche; SARS-CoV-2; clinical evaluation; coronavirus disease; diagnostics; rapid antigen test; respiratory infections; severe acute respiratory syndrome coronavirus 2; the Netherlands; viruses; zoonoses.
Figures
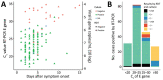
References
-
- World Health Organization. Listings of WHO’s response to COVID-19. 29 June 2020. [cited 2021 Mar 8]. https://www.who.int/news/item/29-06-2020-covidtimeline
-
- World Health Organization. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases. 2020. [cited 2021 Mar 8]. https://www.who.int/publications/i/item/10665-331501
-
- (FINDdx) FIND. SARS-COV-2 diagnostic pipeline. 2020. [cited 2021 Mar 8]. https://www.finddx.org/covid-19/pipeline/
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

