Methyl gallate, gallic acid-derived compound, inhibit cell proliferation through increasing ROS production and apoptosis in hepatocellular carcinoma cells
- PMID: 33725002
- PMCID: PMC7963062
- DOI: 10.1371/journal.pone.0248521
Methyl gallate, gallic acid-derived compound, inhibit cell proliferation through increasing ROS production and apoptosis in hepatocellular carcinoma cells
Abstract
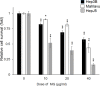
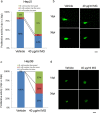
Hepatocellular carcinoma (HCC) is a global health problem. Currently, there is no effective therapeutic strategy for HCC. Methyl gallate (MG), from plant-derived phenolic gallic acid, has exhibited antitumor efficacy. However, the effect of MG on HCC is unclear. In vitro growth activity was detected by a sulforhodamine assay. A zebrafish xenotransplantation was applied to evaluate the inhibitory effect of MG. Reactive oxygen species (ROS) production, autophagy, and lysosome formation were detected by specific dyes. Finally, apoptosis was examined using annexin V-FITC/PI staining and western blot was performed to determine the molecular mechanism. It was demonstrated that MG treatment inhibited the proliferation of Hep3B, Mahlavu, and HepJ5 cells. Xenotransplantation also showed that MG inhibited the growth of Hep3B and HepJ5 cells. MG treatment increased cellular levels of superoxide and oxidative stress. Increases in autophagy and lysosome formation were found after MG treatment. The western blot analysis showed that MG activated cleavage of caspase-3 and poly (SDP ribose) polymerase (PARP), modulated levels of the Bcl2, Bax, and Bad ligands, and induced apoptosis. MG induced autophagy with notable activation of beclin-1, autophagy related 5+12 (ATG5+12), and conversion of light chain 3-I (LC3-I) to II. Our study showed that MG exposure inhibited HCC proliferation both in vitro and in vivo. And blocking autophagy enhanced MG-induced cytotoxicity in HCC cells. These findings suggested MG might serve as a powerful therapeutic supplement for human HCC patients.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Propyl gallate inhibits hepatocellular carcinoma cell growth through the induction of ROS and the activation of autophagy.PLoS One. 2019 Jan 17;14(1):e0210513. doi: 10.1371/journal.pone.0210513. eCollection 2019. PLoS One. 2019. PMID: 30653551 Free PMC article.
-
Autophagy inhibition contributes to the synergistic interaction between EGCG and doxorubicin to kill the hepatoma Hep3B cells.PLoS One. 2014 Jan 21;9(1):e85771. doi: 10.1371/journal.pone.0085771. eCollection 2014. PLoS One. 2014. PMID: 24465696 Free PMC article.
-
Caffeine enhances the anti-tumor effect of 5-fluorouracil via increasing the production of reactive oxygen species in hepatocellular carcinoma.Med Oncol. 2019 Oct 29;36(12):97. doi: 10.1007/s12032-019-1323-8. Med Oncol. 2019. PMID: 31664534
-
The involvement of ROS-regulated programmed cell death in hepatocellular carcinoma.Crit Rev Oncol Hematol. 2024 May;197:104361. doi: 10.1016/j.critrevonc.2024.104361. Epub 2024 Apr 16. Crit Rev Oncol Hematol. 2024. PMID: 38626849 Review.
-
Protective Roles and Therapeutic Effects of Gallic Acid in the Treatment of Cardiovascular Diseases: Current Trends and Future Directions.Curr Med Chem. 2024;31(24):3733-3751. doi: 10.2174/0109298673259299230921150030. Curr Med Chem. 2024. PMID: 37815180 Review.
Cited by
-
Chemical Composition, Antioxidant Activity, and Antibacterial Activity of Black Poplar Buds' Hydroalcoholic Macerates from Dobrogea Area.Molecules. 2023 Jun 22;28(13):4920. doi: 10.3390/molecules28134920. Molecules. 2023. PMID: 37446583 Free PMC article.
-
RNA-Seq Analysis of MCF-7 Breast Cancer Cells Treated with Methyl Gallate Isolated from the Rhizomes of Nymphaea Odorata L. Shows Upregulation of Apoptosis, Autophagy, and Unfolded Protein Canonical Pathways.Molecules. 2025 Jul 18;30(14):3022. doi: 10.3390/molecules30143022. Molecules. 2025. PMID: 40733287 Free PMC article.
-
Anti-Malassezia globosa (MYA-4889, ATCC) activity of Thai propolis from the stingless bee Geniotrigona thoracica.Heliyon. 2024 Apr 16;10(8):e29421. doi: 10.1016/j.heliyon.2024.e29421. eCollection 2024 Apr 30. Heliyon. 2024. PMID: 38660263 Free PMC article.
-
Methyl Gallate Alleviates Acute Ulcerative Colitis by Modulating Gut Microbiota and Inhibiting TLR4/NF-κB Pathway.Int J Mol Sci. 2022 Nov 14;23(22):14024. doi: 10.3390/ijms232214024. Int J Mol Sci. 2022. PMID: 36430509 Free PMC article.
-
Effects of Leea indica leaf extracts and its phytoconstituents on natural killer cell-mediated cytotoxicity in human ovarian cancer.BMC Complement Med Ther. 2023 Mar 11;23(1):79. doi: 10.1186/s12906-023-03904-1. BMC Complement Med Ther. 2023. PMID: 36899361 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

