Accurate point-of-care serology tests for COVID-19
- PMID: 33725025
- PMCID: PMC7963097
- DOI: 10.1371/journal.pone.0248729
Accurate point-of-care serology tests for COVID-19
Abstract
Background: As COVID-19 vaccines become available, screening individuals for prior COVID-19 infection and vaccine response in point-of-care (POC) settings has renewed interest. We prospectively screened at-risk individuals for SARS-CoV-2 spike and nucleocapsid protein antibodies in a POC setting to determine if it was a feasible method to identify antibody from prior infection.
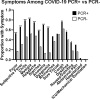
Methods: Three EUA-approved lateral flow antibody assays were performed on POC finger-stick blood and compared with serum and a CLIA nucleocapsid antibody immunoassay. Variables including antibody class, time since PCR, and the assay antigen used were evaluated.
Results: 512 subjects enrolled, of which 104 had a COVID-19 history and positive PCR. Only three PCR-positive subjects required hospitalization, with one requiring mechanical ventilation. The POC results correlated well with the immunoassay (93-97% sensitivity) and using serum did not improve the sensitivity or specificity.
Conclusions: Finger-stick, POC COVID-19 antibody testing was highly effective in identifying antibody resulting from prior infections in mildly symptomatic subjects. Using high-complexity serum immunoassays did not improve the screening outcome. Almost all individuals with COVID-19 infection produced detectable antibodies to the virus. POC antibody testing is useful as a screen for prior COVID-19 infection, and should be useful in assessing vaccine response.
Conflict of interest statement
Dr. Schuler reported salary and other support from the Mary H. Weiser Food Allergy Center and the Taubman Innovation Institute at UM, as well as a UM COVID-19 Innovation Grant; he has also received sponsored project support from Healgen Scientific and Access Bio Inc. Dr. Troost was supported in part by the National Center for Advancing Translational Sciences (NCATS) for the Michigan Institute for Clinical and Health Research (UL1TR002240). Dr. Baldwin and Dr. Baker reported salary support from a UM COVID-19 Innovation Grant and sponsored project support from Healgen Scientific and Access Bio Inc. Dr. Gherasim, Dr. O’Shea, Dr. Manthei, Mr. Chen, and Dr. Giacherio reported no Competing Interests. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


References
-
- WHO. WHO Coronavirus Disease (COVID-19) Dashboard 2021 [updated 2/24/2021]. Available from: https://covid19.who.int/table.
-
- Jarrom D, Elston L, Washington J, Prettyjohns M, Cann K, Myles S, et al.. Effectiveness of tests to detect the presence of SARS-CoV-2 virus, and antibodies to SARS-CoV-2, to inform COVID-19 diagnosis: a rapid systematic review. BMJ Evid Based Med. 2020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

