Purging human ovarian cortex of contaminating leukaemic cells by targeting the mitotic catastrophe signalling pathway
- PMID: 33725274
- PMCID: PMC8266964
- DOI: 10.1007/s10815-021-02081-9
Purging human ovarian cortex of contaminating leukaemic cells by targeting the mitotic catastrophe signalling pathway
Abstract
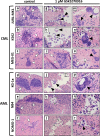
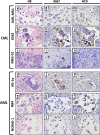
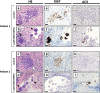
Purpose: Is it possible to eliminate metastasised chronic myeloid leukaemia (CML) and acute myeloid leukaemia (AML) cells from ovarian cortex fragments by inhibition of Aurora B/C kinases (AURKB/C) without compromising ovarian tissue or follicles?
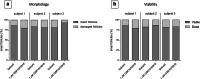
Methods: Human ovarian cortex tissue with experimentally induced tumour foci of CML, AML and primary cells of AML patients were exposed to a 24h treatment with 1 μM GSK1070916, an AURKB/C inhibitor, to eliminate malignant cells by invoking mitotic catastrophe. After treatment, the inhibitor was removed, followed by an additional culture period of 6 days to allow any remaining tumour cells to form new foci. Ovarian tissue integrity after treatment was analysed by four different assays. Appropriate controls were included in all experiments.
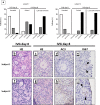
Results: Foci of metastasised CML and AML cells in ovarian cortex tissue were severely affected by a 24h ex vivo treatment with an AURKB/C inhibitor, leading to the formation of multi-nuclear syncytia and large-scale apoptosis. Ovarian tissue morphology and viability was not compromised by the treatment, as no significant difference was observed regarding the percentage of morphologically normal follicles, follicular viability, glucose uptake or in vitro growth of small follicles between ovarian cortex treated with 1 μM GSK1070916 and the control.
Conclusion: Purging of CML/AML metastases in ovarian cortex is possible by targeting the Mitotic Catastrophe Signalling Pathway using GSK1070916 without affecting the ovarian tissue. This provides a therapeutic strategy to prevent reintroduction of leukaemia and enhances safety of autotransplantation in leukaemia patients currently considered at high risk for ovarian involvement.
Keywords: Aurora kinases; Cryopreservation; GSK1070916; Myeloid leukaemia; Ovarian cortex; Purging.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Enhancing the safety of ovarian cortex autotransplantation: cancer cells are purged completely from human ovarian tissue fragments by pharmacological inhibition of YAP/TAZ oncoproteins.Hum Reprod. 2019 Mar 1;34(3):506-518. doi: 10.1093/humrep/dey384. Hum Reprod. 2019. PMID: 30597012
-
Eliminating malignant cells from cryopreserved ovarian tissue is possible in leukaemia patients.Br J Haematol. 2017 Jul;178(2):231-239. doi: 10.1111/bjh.14657. Epub 2017 Apr 17. Br J Haematol. 2017. PMID: 28419412
-
Reversine triggers mitotic catastrophe and apoptosis in K562 cells.Leuk Res. 2016 Sep;48:26-31. doi: 10.1016/j.leukres.2016.06.011. Epub 2016 Jul 1. Leuk Res. 2016. PMID: 27447890
-
The aurora kinases in cell cycle and leukemia.Oncogene. 2015 Jan 29;34(5):537-45. doi: 10.1038/onc.2014.14. Epub 2014 Mar 17. Oncogene. 2015. PMID: 24632603 Free PMC article. Review.
-
(-)-Epigallocatechin-3-gallate induces cell apoptosis in chronic myeloid leukaemia by regulating Bcr/Abl-mediated p38-MAPK/JNK and JAK2/STAT3/AKT signalling pathways.Clin Exp Pharmacol Physiol. 2019 Feb;46(2):126-136. doi: 10.1111/1440-1681.13037. Epub 2018 Oct 30. Clin Exp Pharmacol Physiol. 2019. PMID: 30251267 Review.
Cited by
-
Optimizing treatment efficacy and fertility preservation in patients undergoing hematopoietic stem cell transplantation: A narrative review of ovarian shielding with total-body irradiation or treosulfan-based conditioning regimens.Reprod Med Biol. 2025 Apr 17;24(1):e12648. doi: 10.1002/rmb2.12648. eCollection 2025 Jan-Dec. Reprod Med Biol. 2025. PMID: 40255903 Free PMC article. Review.
-
A comprehensive review and update on human fertility cryopreservation methods and tools.Front Vet Sci. 2023 Apr 18;10:1151254. doi: 10.3389/fvets.2023.1151254. eCollection 2023. Front Vet Sci. 2023. PMID: 37143497 Free PMC article. Review.
-
Minimal Infiltrative Disease Identification in Cryopreserved Ovarian Tissue of Girls with Cancer for Future Use: A Systematic Review.Cancers (Basel). 2023 Aug 22;15(17):4199. doi: 10.3390/cancers15174199. Cancers (Basel). 2023. PMID: 37686475 Free PMC article. Review.
-
De novo design of a nanoregulator for the dynamic restoration of ovarian tissue in cryopreservation and transplantation.J Nanobiotechnology. 2024 Jun 11;22(1):330. doi: 10.1186/s12951-024-02602-5. J Nanobiotechnology. 2024. PMID: 38862987 Free PMC article. Review.
-
Ex vivo purging of cancer cells from ovarian tissue using photodynamic therapy: a novel strategy to restore fertility in leukemia patients.Hum Reprod Open. 2023 Feb 20;2023(2):hoad005. doi: 10.1093/hropen/hoad005. eCollection 2023. Hum Reprod Open. 2023. PMID: 36895885 Free PMC article.
References
-
- Abir R, Aviram A, Feinmesser M, Stein J, Yaniv I, Parnes D, Ben-Haroush A, Meirow D, Rabizadeh E, Fisch B. Ovarian minimal residual disease in chronic myeloid leukaemia. Reprod BioMed Online. 2014;28:255–260. - PubMed
-
- Amorim CA, Shikanov A. The artificial ovary: current status and future perspectives. Future Oncol. 2016;12:2323–2332. - PubMed
-
- Bastings L, Beerendonk CC, Westphal JR, Massuger LF, Kaal SE, van Leeuwen FE, Braat DD, Peek R. Autotransplantation of cryopreserved ovarian tissue in cancer survivors and the risk of reintroducing malignancy: a systematic review. Hum Reprod Update. 2013;19:483–506. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

