Efficacy of immune checkpoint inhibitors in the treatment of non-small cell lung cancer patients with different genes mutation: A meta-analysis
- PMID: 33725808
- PMCID: PMC7969231
- DOI: 10.1097/MD.0000000000019713
Efficacy of immune checkpoint inhibitors in the treatment of non-small cell lung cancer patients with different genes mutation: A meta-analysis
Abstract
Background: Latest clinical trials have proved the better overall survival (OS) for the use of immune checkpoint inhibitors verse chemotherapy in non-small cell lung cancer (NSCLC) patients. However, we still have no clear ideas of the factors which could affect the efficacy of immune checkpoint inhibitors. Cancer, essentially, is a disease related to genes mutation. Therefore, we conducted a systematic review and meta-analysis to compare efficacy of immune checkpoint inhibitors for NSCLC patients with different genes mutation.
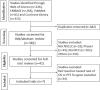
Methods: PubMed, EMBASE, Web of Science, and the Cochrane Library databases were searched for all clinical trials in NSCLC until December 16, 2019. The hazard ratio (HR) and 95% confidence intervals (CIs) of OS or progression-free survival (PFS) were used.
Results: A total of 4453 patients from 7 randomized controlled trials (RCTs) were included. Immune checkpoint inhibitors significantly prolonged the OS (HR, 0.67; 95% CI, 0.60-0.67) in NSCLC patients having epidermal growth factor receptor (EGFR) wild-type versus chemotherapy. Meanwhile, they prolonged the OS (HR, 0.61; 95% CI, 0.39-0.94) in NSCLC patients with Kirsten rat sarcoma viral oncogene homolog (KRAS) mutation. No matter PD-L1 tumor proportion scores were >1% or <1%, immune checkpoint inhibitors were more effective than chemotherapy (HR, 0.64; 95% CI, 0.55-0.75).
Conclusion: Immune checkpoint inhibitors are more efficacious than chemotherapy in NSCLC patients with EGFR wild-type, KRAS mutation, and any PD-L1 tumor proportion scores.
Copyright © 2021 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

