Effects of heavy-load resistance training during (neo-)adjuvant chemotherapy on muscle cellular outcomes in women with breast cancer
- PMID: 33725859
- PMCID: PMC7969308
- DOI: 10.1097/MD.0000000000024960
Effects of heavy-load resistance training during (neo-)adjuvant chemotherapy on muscle cellular outcomes in women with breast cancer
Abstract
Introduction: (Neo-)adjuvant chemotherapy for breast cancer has a deleterious impact on muscle tissue resulting in reduced cardiorespiratory fitness, skeletal muscle mass and function. Physical exercise during treatment may counteract some of these negative effects. However, the effects of resistance training (RT) alone have never been explored. The present study aims to investigate if heavy-load RT during (neo-)adjuvant chemotherapy counteracts deleterious effects on skeletal muscle in women diagnosed with breast cancer. We hypothesize that (neo-)adjuvant treatment with chemotherapy will reduce muscle fiber size, impair mitochondrial function, and increase indicators of cellular stress and that RT during treatment will counteract these negative effects. We also hypothesize that RT during (neo-)adjuvant chemotherapy will increase muscle and blood levels of potential antitumor myokines and reduce treatment-related side effects on muscle strength and cardiorespiratory fitness.
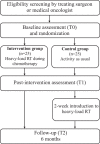
Methods: Fifty women recently diagnosed with breast cancer scheduled to start (neo-)adjuvant chemotherapy will be randomized to either randomized to either intervention group or to control group.The intervention group will perform supervised heavy-load RT twice a week over the course of chemotherapy (approximately 16-weeks) whereas the control group will be encouraged to continue with their usual activities. Muscle biopsies from m. vastus lateralis will be collected before the first cycle of chemotherapy (T0), after chemotherapy (T1), and 6 months later (T2) for assessment of muscle cellular outcomes. The primary outcome for this study is muscle fiber size. Secondary outcomes are: regulators of muscle fiber size and function, indicators of cellular stress and mitochondrial function, myokines with potential antitumor effects, muscle strength, and cardiorespiratory fitness.
Ethics and dissemination: Ethical approval has been obtained from the Regional Ethical Review Board in Uppsala, Sweden (Dnr:2016/230/2). Results will be disseminated through presentations at scientific meetings, publications in peer-reviewed journals, social media, and patient organizations.
Trial registration number: NCT04586517.
Copyright © 2021 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no conflicts of interests to disclose.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical