Development of an indirect ELISA for detection of anti-Mycoplasma hyopneumoniae IgG in naturally infected pathogen-induced convalescent sera
- PMID: 33726780
- PMCID: PMC7968261
- DOI: 10.1186/s12917-021-02828-7
Development of an indirect ELISA for detection of anti-Mycoplasma hyopneumoniae IgG in naturally infected pathogen-induced convalescent sera
Abstract
Background: Immunization of pigs with an inactivated Mycoplasma hyopneumoniae vaccine (bacterin) generates hyperimmune serum that contains high concentrations of anti-M. hyopneumoniae IgG. Commercially available IgG-ELISA kits cannot distinguish between anti-M. hyopneumoniae IgG in inactivated bacterin-induced hyperimmune sera and convalescent sera resulting from natural M. hyopneumoniae infection. Establishment of an ELISA to detect anti-M. hyopneumoniae IgG in convalescent sera will facilitate the evaluation of the M. hyopneumoniae status of pig farms.
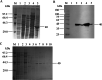
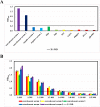
Results: In this study, we expressed and purified recombinant Mhp366-N protein, which contains an epitope recognized by M. hyopneumoniae convalescent sera but not hyperimmune sera, for use as a coating antigen. For the M. hyopneumoniae convalescent serum IgG-ELISA, the optimal antigen concentration, blocking buffer, blocking time, dilution of serum, incubation time with serum, secondary antibody dilution, secondary antibody incubation time and colorimetric reaction time were 0.25 µg/mL, 2.5 % skim milk, 1 h, 1:500, 0.5 h, 1:10,000, 1 h and 15 min, respectively. Validation of the M. hyopneumoniae convalescent serum IgG-ELISA showed a cut-off value of 0.323, the intra-assay CV ranged from 3.27 to 7.26 %, the inter-assay CV ranged from 3.46 to 5.93 %, and the assay was able to differentiate convalescent sera from antibodies to 7 other porcine respiratory pathogens. The convalescent serum IgG-ELISA detected no anti-M. hyopneumoniae IgG in hyperimmune serum samples while a commercial IgG-ELISA identified 95/145 of these sera as positive. The accuracy of the M. hyopneumoniae convalescent serum IgG-ELISA was comparable to the sIgA-ELISA but better than the commercial IgG-ELISA.
Conclusions: The convalescent serum IgG-ELISA is a reproducible, sensitive, and specific indirect ELISA to detect anti-M. hyopneumoniae IgG in naturally infected pathogen-induced convalescent sera. This ELISA could be used to carry out large scale surveillance of M. hyopneumoniae infection in pig farms regardless of vaccination status.
Keywords: Convalescent sera; IgG; Indirect ELISA; Mhp366; Mycoplasma hyopneumoniae.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Maes D, Sibila M, Kuhnert P, Segalés J, Haesebrouck F, Pieters M. Update on Mycoplasma hyopneumoniae infections in pigs: Knowledge gaps for improved disease control. Transbound Emerg Dis. 2018; Suppl 1: 110–24. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

