A U.S. isolate of Theileria orientalis, Ikeda genotype, is transmitted to cattle by the invasive Asian longhorned tick, Haemaphysalis longicornis
- PMID: 33726815
- PMCID: PMC7962341
- DOI: 10.1186/s13071-021-04659-9
A U.S. isolate of Theileria orientalis, Ikeda genotype, is transmitted to cattle by the invasive Asian longhorned tick, Haemaphysalis longicornis
Abstract
Background: Theileria orientalis is a tick-borne hemoparasite that causes anemia, ill thrift, and death in cattle globally. The Ikeda strain of T. orientalis is more virulent than other strains, leading to severe clinical signs and death of up to 5% of affected animals. Within the Asia-Pacific region, where it affects 25% of Australian cattle, T. orientalis Ikeda has a significant economic impact on the cattle industry. In 2017, T. orientalis Ikeda was detected in a cattle herd in Albermarle County, Virginia, United States. Months earlier, the U.S. was alerted to the invasion of the Asian longhorned tick, Haemaphysalis longicornis, throughout the eastern U.S. Abundant H. longicornis ticks were identified on cattle in the T. orientalis-affected herd in VA, and a subset of ticks from the environment were PCR-positive for T. orientalis Ikeda. A strain of T. orientalis from a previous U.S. outbreak was not transmissible by H. longicornis; however, H. longicornis is the primary tick vector of T. orientalis Ikeda in other regions of the world. Thus, the objective of this study was to determine whether invasive H. longicornis ticks in the U.S. are competent vectors of T. orientalis Ikeda.
Methods: Nymphal H. longicornis ticks were fed on a splenectomized calf infected with the VA-U.S.-T. orientalis Ikeda strain. After molting, a subset of adult ticks from this cohort were dissected, and salivary glands assayed for T. orientalis Ikeda via qPCR. The remaining adult ticks from the group were allowed to feed on three calves. Calves were subsequently monitored for T. orientalis Ikeda infection via blood smear cytology and PCR.
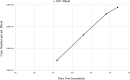
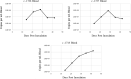
Results: After acquisition feeding on a VA-U.S.-T. orientalis Ikeda-infected calf as nymphs, a subset of molted adult tick salivary glands tested positive by qPCR for T. orientalis Ikeda. Adult ticks from the same cohort successfully transmitted T. orientalis Ikeda to 3/3 naïve calves, each of which developed parasitemia reaching 0.4-0.9%.
Conclusions: Our findings demonstrate that U.S. H. longicornis ticks are competent vectors of the VA-U.S.-T. orientalis Ikeda strain. This data provides important information for the U.S. cattle industry regarding the potential spread of this parasite and the necessity of enhanced surveillance and control measures.
Keywords: Asian longhorned tick; Cattle; Haemaphysalis longicornis; Ikeda genotype; Theileria orientalis; Transmission.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Eamens GJ, Gonsalves JR, Jenkins C, Collins D, Bailey G. Theileria orientalis MPSP types in Australian cattle herds associated with outbreaks of clinical disease and their association with clinical pathology findings. Vet Parasitol. 2013;191(3–4):209–17. doi: 10.1016/j.vetpar.2012.09.007. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

