Expert consensus statements for the management of COVID-19-related acute respiratory failure using a Delphi method
- PMID: 33726819
- PMCID: PMC7962430
- DOI: 10.1186/s13054-021-03491-y
Expert consensus statements for the management of COVID-19-related acute respiratory failure using a Delphi method
Abstract
Background: Coronavirus disease 2019 (COVID-19) pandemic has caused unprecedented pressure on healthcare system globally. Lack of high-quality evidence on the respiratory management of COVID-19-related acute respiratory failure (C-ARF) has resulted in wide variation in clinical practice.
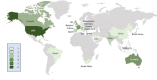
Methods: Using a Delphi process, an international panel of 39 experts developed clinical practice statements on the respiratory management of C-ARF in areas where evidence is absent or limited. Agreement was defined as achieved when > 70% experts voted for a given option on the Likert scale statement or > 80% voted for a particular option in multiple-choice questions. Stability was assessed between the two concluding rounds for each statement, using the non-parametric Chi-square (χ2) test (p < 0·05 was considered as unstable).
Results: Agreement was achieved for 27 (73%) management strategies which were then used to develop expert clinical practice statements. Experts agreed that COVID-19-related acute respiratory distress syndrome (ARDS) is clinically similar to other forms of ARDS. The Delphi process yielded strong suggestions for use of systemic corticosteroids for critical COVID-19; awake self-proning to improve oxygenation and high flow nasal oxygen to potentially reduce tracheal intubation; non-invasive ventilation for patients with mixed hypoxemic-hypercapnic respiratory failure; tracheal intubation for poor mentation, hemodynamic instability or severe hypoxemia; closed suction systems; lung protective ventilation; prone ventilation (for 16-24 h per day) to improve oxygenation; neuromuscular blocking agents for patient-ventilator dyssynchrony; avoiding delay in extubation for the risk of reintubation; and similar timing of tracheostomy as in non-COVID-19 patients. There was no agreement on positive end expiratory pressure titration or the choice of personal protective equipment.
Conclusion: Using a Delphi method, an agreement among experts was reached for 27 statements from which 20 expert clinical practice statements were derived on the respiratory management of C-ARF, addressing important decisions for patient management in areas where evidence is either absent or limited.
Trial registration: The study was registered with Clinical trials.gov Identifier: NCT04534569.
Keywords: COVID 19 invasive mechanical ventilation; COVID-19 acute respiratory distress syndrome; COVID-19 high flow nasal oxygen; COVID-19 respiratory management; COVID-19 ventilatory management; Respiratory distress syndrome adult.
Conflict of interest statement
EA reports having taken professional fees for lectures from Gilead, Pfizer, Baxter and Alexion. His research group has been supported by Ablynx, Fisher & Paykel, Jazz Pharma and MSD, all outside the scope of submitted work. AKK reports institutional funding for 2 trials, a randomized Clinical Trial of CLR2.0 Hemofiltration Treatment (C2Rx) in Severe or Critically Ill Adults With COVID-19 Infection (ClinicalTrials.gov Identifier: NCT04537975) and Blood Volume Assessment in COVID-19 ICU Patients—BVAC19 (ClinicalTrials.gov Identifier: NCT04517695). He is site PI for SCCM Discovery Network Viral Infection and Respiratory Illness Universal Study [VIRUS]: COVID-19 Registry (ClinicalTrials.gov Identifier: NCT04323787) and is a member of American Society of Anesthesiologists (ASA) COVID-19 task force. AKK is a key opinion leader and consults for Medtronic, Edwards Lifesciences, Philips North America and Zoll Medical, is on the advisory board for Potrero Medical and Retia Medical and receives compensation for his position for the chair of the trial steering committee for the SILtuximab in Viral ARds (SILVAR) Study (SILVAR) (ClinicalTrials.gov Identifier: NCT04616586) all outside the scope of the submitted work. He is also funded with a Clinical and Translational Science Institute (CTSI) NIH/NCTAS KL2 TR001421 award for a trial on continuous postoperative hemodynamic and saturation monitoring. AKK is a founding member of BrainX LLC, a collaborative platform for research and development of artificial intelligence technology in critical care and perioperative medicine. YJ is a member of CII Medical Task Force, India and a member of the steering committee for a Phase 3, Prospective, Randomized, Open Label, Comparative, Clinical Study To Evaluate Efficacy And Safety Of Ulinastatin Plus Standard-Of-Care Compared To Standard-Of-Care In Treatment Of Acute Respiratory Distress Syndrome (ARDS) In Hospitalized COVID-19 Infection Patients. WAH is a co-chair of COVID-19 surviving sepsis campaign guidelines. MA is a panel member of Surviving Sepsis Campaign. YMA is a co-investigator on COVI-PRONE trial (ClinicalTrials.gov Identifier: NCT04350723) and is a panel member of Surviving Sepsis Campaign CPG. LJB reports grants from Medtronic Covidien, grants and non-financial support from Fisher Paykel, non-financial support from Air Liquide, Sentec, Philips and General Electric (patent), all outside the scope of submitted work. SJ reports receiving consulting fees from Drager, Medtronic, Baxter, Fresenius and Fisher & Paykel, all outside the scope of submitted work. JBL received lectures fees from BD and Zoll (outside the scope: cooling devices), all outside the scope of submitted work. MLNGM is a co-founder, past-President and current Treasurer of WSACS (The Abdominal Compartment Society,
Figures

References
-
- Kim L, Garg S, O'Halloran A, Whitaker M, Pham H, Anderson EJ, et al. Risk Factors for Intensive Care Unit Admission and In-hospital Mortality among Hospitalized Adults Identified through the U.S. Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET). Clin Infect Dis. 2020: ciaa1012. - PMC - PubMed
-
- European Centre for Disease Prevention and Control (ECDC). COVID-19 Surveillance Report (Week 44, 2020). https://covid19-surveillance-report.ecdc.europa.eu Accessed 11 November 2020.
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

