EPA guidance on treatment of negative symptoms in schizophrenia
- PMID: 33726883
- PMCID: PMC8057437
- DOI: 10.1192/j.eurpsy.2021.13
EPA guidance on treatment of negative symptoms in schizophrenia
Abstract
Negative symptoms of schizophrenia remain a major therapeutic challenge. The progress in the conceptualization and assessment is not yet fully reflected by treatment research. Nevertheless, there is a growing evidence base regarding the effects of biological and psychosocial interventions on negative symptoms. The importance of the distinction between primary and secondary negative symptoms for treatment selection might seem evident, but the currently available evidence remains limited. Good clinical practice is recommended for the treatment of secondary negative symptoms. Antipsychotic treatment should be optimized to avoid secondary negative symptoms due to side effects and due to positive symptoms. For most available interventions, further evidence is needed to formulate sound recommendations for primary, persistent, or predominant negative symptoms.However, based on currently available evidence recommendations for the treatment of undifferentiated negative symptoms (including both primary and secondary negative symptoms) are provided. Although it has proven difficult to formulate an evidence-based recommendation for the choice of an antipsychotic, a switch to a second-generation antipsychotic should be considered for patients who are treated with a first-generation antipsychotic. Antidepressant add-on to antipsychotic treatment is an option. Social skills training is recommended as well as cognitive remediation for patients who also show cognitive impairment. Exercise interventions also have shown promise. Finally, access to treatment and to psychosocial rehabilitation should be ensured for patients with negative symptoms. Overall, there is definitive progress in the field, but further research is clearly needed to develop specific treatments for negative symptoms.
Keywords: Negative symptoms; schizophrenia; treatment.
Conflict of interest statement
S. Galderisi has been a consultant and/or advisor to or has received honoraria or grants from Millennium Pharmaceuticals, Innova Pharma-Recordati Group, Janssen Pharmaceutica NV, Sunovion Pharmarmaceuticals, Janssen-Cilag Polska Sp. zo. o., Gedeon Richter-Recordati, Pierre Fabre, Otsuka, and Angelini.
A. Mucci received honoraria, advisory board, or consulting fees from the following companies: Amgen Dompé, Angelini-Acraf, Astra Zeneca, Bristol-Myers Squibb, Gedeon Richter Bulgaria, Innova-Pharma, Janssen Pharmaceuticals, Lundbeck, Otsuka, Pfizer, and Pierre Fabre.
S. Dollfus received honoraria as expert/consultant by Fabre, Gedeon, Roche, and Takeda; invited Conferences: Lundbeck, Otsuka, Janssen, and has contracts with Prophase MedAvances and NeuroCogTrials.
S. Kaiser has royalties for cognitive training software from Schuhfried and advisory board honoraria from Recordati and Lundbeck on an institutional account for education and research.
I. Bitter has received in the past 5 years honoraria or consultation fees from Angelini, Eli Lilly, Gedeon Richter, Janssen/Janssen Cilag, and Sun Pharma.
All other authors declare no conflict of interest.
Figures
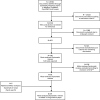
References
-
- Bleuler E. Dementia praecox or the group of schizophrenias. New York, NY: International Universities Press; 1950.
-
- Jackson JH. Selected writings of JH Jackson. London, UK: Hodder and Stoughton; 1931.
-
- Kraepelin E. Dementia praecox and paraphrenia. Chicago, IL: Chicago Medical Book Company; 1919.
-
- Galderisi S, Mucci A, Buchanan RW, Arango C. Negative symptoms of schizophrenia: new developments and unanswered research questions. Lancet Psychiatry. 2018;5(8):664–677. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

