Multigenerational nicotine exposure affects offspring nicotine metabolism, nicotine-induced hypothermia, and basal corticosterone in a sex-dependent manner
- PMID: 33727150
- PMCID: PMC8137648
- DOI: 10.1016/j.ntt.2021.106972
Multigenerational nicotine exposure affects offspring nicotine metabolism, nicotine-induced hypothermia, and basal corticosterone in a sex-dependent manner
Abstract
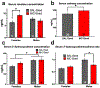
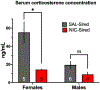
Parental nicotine exposure can impact phenotypes in unexposed offspring. Our laboratory recently published data showing that nicotine reward and hippocampal gene expression involved in stress pathways were perturbed in F1 offspring of male C57BL/6J mice chronically exposed to nicotine. For the current study, we aimed to further test nicotine and stress-sensitivity phenotypes that may predict vulnerability to nicotine addiction in new cohorts of F1 offspring derived from nicotine-exposed males. We tested locomotor and body temperature sensitivity to acute nicotine administration, serum concentration of nicotine and nicotine metabolites after acute nicotine dosing, and serum corticosterone levels in male and female F1 offspring of nicotine- or saline-exposed males. Paternal nicotine exposure reduced sensitivity to nicotine-induced hypothermia in males, altered nicotine metabolite concentrations in males and females, and reduced serum basal corticosterone levels in females. These findings may point to reduced susceptibility to nicotine addiction-related phenotypes as a result of parental nicotine exposure.
Keywords: Acetylcholine; Addiction; Genetic; Intergenerational inheritance; Metabolism; Stress.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Chronic nicotine exposure alters sperm small RNA content in C57BL/6J mouse model.Dev Psychobiol. 2023 Mar;65(2):e22367. doi: 10.1002/dev.22367. Dev Psychobiol. 2023. PMID: 36811365 Free PMC article. Review.
-
Paternal nicotine enhances fear memory, reduces nicotine administration, and alters hippocampal genetic and neural function in offspring.Addict Biol. 2021 Jan;26(1):e12859. doi: 10.1111/adb.12859. Epub 2019 Nov 28. Addict Biol. 2021. PMID: 31782218 Free PMC article.
-
Adolescent chronic variable social stress influences exploratory behavior and nicotine responses in male, but not female, BALB/cJ mice.Brain Res Bull. 2018 Apr;138:37-49. doi: 10.1016/j.brainresbull.2017.08.001. Epub 2017 Aug 9. Brain Res Bull. 2018. PMID: 28802900 Free PMC article.
-
Paternal nicotine exposure in rats produces long-lasting neurobehavioral effects in the offspring.Neurotoxicol Teratol. 2019 Jul-Aug;74:106808. doi: 10.1016/j.ntt.2019.05.001. Epub 2019 May 16. Neurotoxicol Teratol. 2019. PMID: 31103693 Free PMC article.
-
Multigenerational and transgenerational effects of paternal exposure to drugs of abuse on behavioral and neural function.Eur J Neurosci. 2019 Aug;50(3):2453-2466. doi: 10.1111/ejn.14060. Epub 2018 Jul 20. Eur J Neurosci. 2019. PMID: 29949212 Free PMC article. Review.
Cited by
-
Adolescent intermittent alcohol exposure produces strain-specific cross-sensitization to nicotine and other behavioral adaptations in adulthood in C57BL/6J and DBA/2J mice.Pharmacol Biochem Behav. 2023 Nov;232:173655. doi: 10.1016/j.pbb.2023.173655. Epub 2023 Oct 5. Pharmacol Biochem Behav. 2023. PMID: 37802393 Free PMC article.
-
Age is associated with altered locomotor and hypothermic response to acute nicotine.Behav Pharmacol. 2025 Feb 1;36(1):60-69. doi: 10.1097/FBP.0000000000000804. Epub 2024 Dec 12. Behav Pharmacol. 2025. PMID: 39660850
-
Chronic nicotine exposure alters sperm small RNA content in C57BL/6J mouse model.Dev Psychobiol. 2023 Mar;65(2):e22367. doi: 10.1002/dev.22367. Dev Psychobiol. 2023. PMID: 36811365 Free PMC article. Review.
-
Influence of substance use on male reproductive health and offspring outcomes.Nat Rev Urol. 2024 Sep;21(9):534-564. doi: 10.1038/s41585-024-00868-w. Epub 2024 Apr 25. Nat Rev Urol. 2024. PMID: 38664544 Review.
-
Extended abstinence from morphine alters sperm smRNA expression and prevents transmission of intergenerational phenotypes.Environ Epigenet. 2025 Mar 20;11(1):dvaf006. doi: 10.1093/eep/dvaf006. eCollection 2025. Environ Epigenet. 2025. PMID: 40406632 Free PMC article.
References
-
- Gill KJ, & Boyle AE (2005). Genetic basis for the psychostimulant effects of nicotine: a quantitative trait locus analysis in AcB/BcA recombinant congenic mice. Genes, Brain and Behavior, 4(7), 401–411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

