Intracellular detection and communication of a wireless chip in cell
- PMID: 33727598
- PMCID: PMC7966781
- DOI: 10.1038/s41598-021-85268-5
Intracellular detection and communication of a wireless chip in cell
Abstract
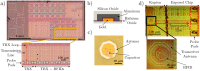
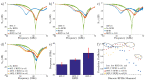
The rapid growth and development of technology has had significant implications for healthcare, personalized medicine, and our understanding of biology. In this work, we leverage the miniaturization of electronics to realize the first demonstration of wireless detection and communication of an electronic device inside a cell. This is a significant forward step towards a vision of non-invasive, intracellular wireless platforms for single-cell analyses. We demonstrate that a 25 [Formula: see text]m wireless radio frequency identification (RFID) device can not only be taken up by a mammalian cell but can also be detected and specifically identified externally while located intracellularly. The S-parameters and power delivery efficiency of the electronic communication system is quantified before and after immersion in a biological environment; the results show distinct electrical responses for different RFID tags, allowing for classification of cells by examining the electrical output noninvasively. This versatile platform can be adapted for realization of a broad modality of sensors and actuators. This work precedes and facilitates the development of long-term intracellular real-time measurement systems for personalized medicine and furthering our understanding of intrinsic biological behaviors. It helps provide an advanced technique to better assess the long-term evolution of cellular physiology as a result of drug and disease stimuli in a way that is not feasible using current methods.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

