Increased Nuclear Transporter KPNA2 Contributes to Tumor Immune Evasion by Enhancing PD-L1 Expression in PDAC
- PMID: 33728352
- PMCID: PMC7939744
- DOI: 10.1155/2021/6694392
Increased Nuclear Transporter KPNA2 Contributes to Tumor Immune Evasion by Enhancing PD-L1 Expression in PDAC
Abstract
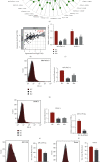
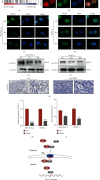
Pancreatic ductal adenocarcinoma (PDAC) is one of the deadliest malignancies and is known for its high resistance and low response to treatment. Tumor immune evasion is a major stumbling block in designing effective anticancer therapeutic strategies. Karyopherin alpha 2 (KPNA2), a member of the nuclear transporter family, is elevated in multiple human cancers and accelerates carcinogenesis. However, the specific role of KPNA2 in PDAC remains unclear. In this study, we found that expression of KPNA2 was significantly upregulated in PDAC compared to adjacent nontumor tissue and its high expression was correlated with poor survival outcome by analyzing the GEO datasets. Similar KPNA2 expression pattern was also found in both human patient samples and KPC mouse models through IHC staining. Although KPNA2 knockdown failed to impair the vitality and migration ability of PDAC cells in vitro, the in vivo tumor growth was significantly impeded and the expression of immune checkpoint ligand PD-L1 was reduced by silencing KPNA2. Furthermore, we uncovered that KPNA2 modulated the expression of PD-L1 by mediating nuclear translocation of STAT3. Collectively, our data suggested that KPNA2 has the potential to serve as a promising biomarker for diagnosis in PDAC.
Copyright © 2021 Kai-Xia Zhou et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

