Ischemic stroke, hemorrhage, and mortality in patients with non-valvular atrial fibrillation and renal dysfunction treated with rivaroxaban: sub-analysis of the EXPAND study
- PMID: 33728513
- PMCID: PMC8332581
- DOI: 10.1007/s00380-021-01810-5
Ischemic stroke, hemorrhage, and mortality in patients with non-valvular atrial fibrillation and renal dysfunction treated with rivaroxaban: sub-analysis of the EXPAND study
Abstract
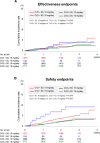
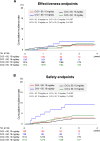
The EXPAND Study demonstrated the effectiveness and safety of rivaroxaban in patients with non-valvular atrial fibrillation (NVAF) in routine clinical practice in Japan. This sub-analysis was conducted to reveal the effectiveness and safety of rivaroxaban in Japanese NVAF patients according to baseline creatinine clearance (CrCl) levels and rivaroxaban doses in the EXPAND Study. We examined 6806 patients whose baseline CrCl data were available and classified them into 2 groups: normal renal function group with CrCl ≥ 50 mL/min (n = 5326, 78%) and renal dysfunction group with CrCl < 50 mL/min (n = 1480, 22%). In the normal renal function group, 1609 (30%) received 10 mg/day (under-dose), while in the renal dysfunction group, 108 (7%) received 15 mg/day (over-dose). In the normal renal function group, under-dose of rivaroxaban was associated with higher all-cause mortality, while in the renal dysfunction group, over-dose was associated with higher incidence of major bleeding. In contrast, the incidence of stroke or systemic embolism was not different between the 2 groups regardless of the dose of rivaroxaban. In the propensity score matched analysis to adjust the difference in characteristics according to doses of rivaroxaban, the incidences of clinical outcomes were comparable between the 2 dose groups in both renal function groups. These results indicate that the dose of rivaroxaban should be reduced depending on the renal function, considering the balance between risks of bleeding and ischemia.
Keywords: Creatinine clearance; Non-valvular atrial fibrillation; Renal dysfunction; Rivaroxaban.
© 2021. The Author(s).
Conflict of interest statement
H.A. has received personal fee from Daiichi Sankyo, outside the submitted work. S.U. has received personal fees from Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, Sanofi, Dainippon Sumitomo, Otsuka, Takeda, Astellas, AstraZeneka, Sanwa Kagaku, Shionogi, Mitsubishi Tanabe, and Pfizer, outside the submitted work. H.I. has received personal fees from Bayer Healthcare, Boehringer Ingelheim, Daiichi-Sankyo, and Bristol-Myers Squibb, outside the submitted work. T.K. has received grants and personal fees from Daiichi Sankyo, Bayer Yakuhin, Pfizer, Chugai, Boehringer Ingelheim, Mitsubishi Tanabe, Shionogi, Astellas, and MSD; personal fees from Bristol-Myers Squibb, Sanofi, and AstraZeneca; and grants from Takeda, Kissei, Kyowa Hakko Kirin, EA Pharma, Asahi Kasei Medical, Otsuka, Torii, Eisai, Ono, Zeria, and Dainippon Sumitomo, outside the submitted work. T.Y. has received grants and personal fees from Bayer, Daiichi Sankyo, Bristol-Myers Squibb, and Mitsubishi Tanabe; and personal fees from Pfizer, Eisai, Ono Pharmaceutical, Toa Eiyo, and Nippon Boehringer, outside the submitted work. W.S. has received grants and personal fees from Bayer, Daiichi Sankyo, Nippon Boehringer, Bristol-Myers Squibb, Pfizer, Eisai, Ono Pharmaceutical, and Mitsubishi Tanabe, outside the submitted work. T.I reports grants and personal fees from Daiichi-Sankyo, personal fees from Bayer, grants and personal fees from Bristol-Myers Squibb, personal fees from Pfizer, grants from Boehringer Ingelheim, outside the submitted work. M.K. has received personal fees from Tohoku University, during the conduct of the study; and personal fees from Bayer, outside the submitted work. K.K. has received grants from Bayer Yakuhin, Ltd., Daiichi-Sankyo Co., Ltd.; and honoraria from Bayer Yakuhin, Ltd, and Daiichi-Sankyo Co., Ltd., outside the submitted work. K.F. has received personal fees from Bayer, outside the submitted work. H.O. has received personal fees from Daiichi-Sankyo and Bayer, outside the submitted work. H.S. has received personal fees from Bayer, and Daiichi Sankyo, outside the submitted work.
Figures
Similar articles
-
Clinical risk factors of stroke and major bleeding in patients with non-valvular atrial fibrillation under rivaroxaban: the EXPAND Study sub-analysis.Heart Vessels. 2019 Nov;34(11):1839-1851. doi: 10.1007/s00380-019-01425-x. Epub 2019 May 24. Heart Vessels. 2019. PMID: 31127325 Free PMC article.
-
Comparisons of effectiveness and safety between on-label dosing, off-label underdosing, and off-label overdosing in Asian and non-Asian atrial fibrillation patients treated with rivaroxaban: a systematic review and meta-analysis of observational studies.Europace. 2023 Oct 5;25(10):euad288. doi: 10.1093/europace/euad288. Europace. 2023. PMID: 37738425 Free PMC article.
-
Creatinine clearance and inappropriate dose of rivaroxaban in Japanese patients with non-valvular atrial fibrillation.Heart Vessels. 2020 Jan;35(1):110-117. doi: 10.1007/s00380-019-01457-3. Epub 2019 Jun 20. Heart Vessels. 2020. PMID: 31222552
-
Clinical Risk Factors of Thromboembolic and Major Bleeding Events for Patients with Atrial Fibrillation Treated with Rivaroxaban in Japan.J Stroke Cerebrovasc Dis. 2020 Apr;29(4):104584. doi: 10.1016/j.jstrokecerebrovasdis.2019.104584. Epub 2020 Jan 23. J Stroke Cerebrovasc Dis. 2020. PMID: 31983518
-
Rivaroxaban in the Prevention of Stroke and Systemic Embolism in Patients with Non-Valvular Atrial Fibrillation: Clinical Implications of the ROCKET AF Trial and Its Subanalyses.Am J Cardiovasc Drugs. 2015 Dec;15(6):395-401. doi: 10.1007/s40256-015-0127-2. Am J Cardiovasc Drugs. 2015. PMID: 26062914 Review.
Cited by
-
Outcomes of 10 mg Rivaroxaban in Nonvalvular Atrial Fibrillation Patients With CrCl ≥ 50 mL/min: A Retrospective Cohort Study.Cardiovasc Ther. 2025 Jun 26;2025:7021330. doi: 10.1155/cdr/7021330. eCollection 2025. Cardiovasc Ther. 2025. PMID: 40612113 Free PMC article.
-
Clinical outcomes of off-label DOAC underdosing in Japanese patients with atrial fibrillation: a systematic review and meta-analysis.J Thromb Thrombolysis. 2025 Aug;58(6):709-720. doi: 10.1007/s11239-025-03107-0. Epub 2025 May 29. J Thromb Thrombolysis. 2025. PMID: 40442451 Review.
-
Electroacupuncture pretreatment alleviates rats cerebral ischemia-reperfusion injury by inhibiting ferroptosis.Heliyon. 2024 Apr 26;10(9):e30418. doi: 10.1016/j.heliyon.2024.e30418. eCollection 2024 May 15. Heliyon. 2024. PMID: 38807610 Free PMC article.
-
Relation between laxative use and risk of major bleeding in patients with atrial fibrillation and heart failure.Heart Vessels. 2023 Jul;38(7):938-948. doi: 10.1007/s00380-023-02249-6. Epub 2023 Feb 17. Heart Vessels. 2023. PMID: 36799967 Free PMC article.
-
Plasma Rivaroxaban Level in Patients With Early Stages of Chronic Kidney Disease-Relationships With Renal Function and Clinical Events.Front Pharmacol. 2022 May 17;13:888660. doi: 10.3389/fphar.2022.888660. eCollection 2022. Front Pharmacol. 2022. PMID: 35662694 Free PMC article.
References
-
- Inoue H, Fujiki A, Origasa H, Ogawa S, Okumura K, Kubota I, Aizawa Y, Yamashita T, Atarashi H, Horie M, Ohe T, Doi Y, Shimizu A, Chishaki A, Saikawa T, Yano K, Kitabatake A, Mitamura H, Kodama I, Kamakura S. Prevalence of atrial fibrillation in the general population of Japan: an analysis based on periodic health examination. Int J Cardiol. 2009;137:102–107. doi: 10.1016/j.ijcard.2008.06.029. - DOI - PubMed
-
- Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan-Schilling V, Rowell N, Sinnaeve P, Collins R, Camm AJ, Heidbuchel H, Group ESCSD The 2018 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39:1330–1393. doi: 10.1093/eurheartj/ehy136. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

