Detection of Streptococcus equi subsp. equi in guttural pouch lavage samples using a loop-mediated isothermal nucleic acid amplification microfluidic device
- PMID: 33728675
- PMCID: PMC8163136
- DOI: 10.1111/jvim.16105
Detection of Streptococcus equi subsp. equi in guttural pouch lavage samples using a loop-mediated isothermal nucleic acid amplification microfluidic device
Abstract
Background: Rapid point-of-care (POC) detection of Streptococcus equi subsp. equi (S. equi) would theoretically reduce the spread of strangles by identifying index and carrier horses.
Hypothesis: That the eqbE isothermal amplification (LAMP) assay, and the same eqbE LAMP assay tested in a microfluidic device format, are comparable to a triplex real-time quantitative polymerase chain reaction (qPCR) assay that is commonly used in diagnostic labs.
Samples: Sixty-eight guttural pouch lavage (GPL) specimens from horses recovering from strangles.
Methods: Guttural pouch lavage specimens were tested for S. equi retrospectively using the benchtop eqbE LAMP, the eqbE LAMP microfluidic device, and compared to the triplex qPCR, that detects 2 S. equi-specific genes, eqbE and SEQ2190, as the reference standard using the receiver operating characteristic area under the curve (ROC).
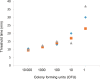
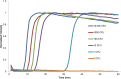
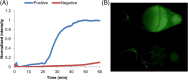
Results: The 27/68 specimens were positive by benchtop eqbE LAMP, 31/64 by eqbE LAMP microfluidic device, and 12/67 by triplex qPCR. Using the triplex PCR as the reference, the benchtop eqbE LAMP showed excellent discrimination (ROC Area = 0.813, 95% confidence interval [CI] = 0.711-0.915) as did the LAMP microfluidic device (ROC Area = 0.811, 95% CI = 0.529-0.782). There was no significant difference between the benchtop LAMP and LAMP microfluidic device (ROC Area 0.813 ± 0.055 vs 0.811 ± 0.034, P = .97).
Conclusions: The eqbE LAMP microfluidic device detected S. equi in GPL specimens from convalescent horses. This assay shows potential for development as a POC device for rapid, sensitive, accurate, and cost-efficient detection of S. equi.
Keywords: DNA amplification; Streptococcus equi; diagnostics; equine; point-of-care; strangles.
© 2021 The Authors. Journal of Veterinary Internal Medicine published by Wiley Periodicals LLC on behalf of American College of Veterinary Internal Medicine.
Conflict of interest statement
The University of Pennsylvania has applied for patent protection for the microfluidic device technology, with H. H. Bau and J. Song named as coinventors. No other authors have a conflict of interest.
Figures


Similar articles
-
Enhancement of loop-mediated isothermal amplification (LAMP) with guanidine hydrochloride for the detection of Streptococcus equi subspecies equi (Strangles).PeerJ. 2024 Oct 8;12:e17955. doi: 10.7717/peerj.17955. eCollection 2024. PeerJ. 2024. PMID: 39421427 Free PMC article.
-
Comparison of nasopharyngeal and guttural pouch specimens to determine the optimal sampling site to detect Streptococcus equi subsp equi carriers by DNA amplification.BMC Vet Res. 2017 Mar 23;13(1):75. doi: 10.1186/s12917-017-0989-4. BMC Vet Res. 2017. PMID: 28335829 Free PMC article.
-
Validation of a point-of-care polymerase chain reaction assay for detection of Streptococcus equi subspecies equi in rostral nasal swabs from horses with suspected strangles.Can Vet J. 2021 Jan;62(1):51-54. Can Vet J. 2021. PMID: 33390599 Free PMC article.
-
Update on Streptococcus equi subsp equi infections.Vet Clin North Am Equine Pract. 2015 Apr;31(1):27-41. doi: 10.1016/j.cveq.2014.11.003. Epub 2015 Jan 16. Vet Clin North Am Equine Pract. 2015. PMID: 25600455 Review.
-
Strangles: taking steps towards eradication.Vet Microbiol. 2013 Nov 29;167(1-2):50-60. doi: 10.1016/j.vetmic.2013.03.033. Epub 2013 Apr 12. Vet Microbiol. 2013. PMID: 23642414 Review.
Cited by
-
Comparative analysis of loop-mediated isothermal amplification combined with microfluidic chip technology and q-PCR in the detection of clinical infectious pathogens.J Clin Lab Anal. 2022 Aug;36(8):e24565. doi: 10.1002/jcla.24565. Epub 2022 Jun 26. J Clin Lab Anal. 2022. PMID: 35754145 Free PMC article.
-
Enhancement of loop-mediated isothermal amplification (LAMP) with guanidine hydrochloride for the detection of Streptococcus equi subspecies equi (Strangles).PeerJ. 2024 Oct 8;12:e17955. doi: 10.7717/peerj.17955. eCollection 2024. PeerJ. 2024. PMID: 39421427 Free PMC article.
-
Comparison of PCR-HRM, colorimetric LAMP and culture based diagnostic assays in the detection of endometritis caused by Streptococcus equi subsp. zooepidemicus in mares.Vet Res Commun. 2023 Jun;47(2):495-509. doi: 10.1007/s11259-022-10047-0. Epub 2022 Dec 20. Vet Res Commun. 2023. PMID: 36538151 Free PMC article.
-
Current and Future Advances in the Detection and Surveillance of Biosecurity-Relevant Equine Bacterial Diseases Using Loop-Mediated Isothermal Amplification (LAMP).Animals (Basel). 2023 Aug 18;13(16):2663. doi: 10.3390/ani13162663. Animals (Basel). 2023. PMID: 37627456 Free PMC article. Review.
References
-
- Newton JR, Verheyen K, Talbot NC, et al. Control of strangles outbreaks by isolation of guttural pouch carriers identified using PCR and culture of Streptococcus equi . Equine Vet J. 2000;32:515‐526. - PubMed
-
- Timoney JF, Artiushin SC. Detection of Streptococcus equi in equine nasal swabs and washes by DNA amplification. Vet Rec. 1997;141:446‐447. - PubMed
-
- Meehan M, Lewis MJ, Byrne C, et al. Localization of the equine IgG‐binding domain in the fibrinogen‐binding protein (FgBP) of Streptococcus equi subsp. equi . Microbiology. 2009;155(pt 8):2583‐2592. - PubMed
-
- Baverud V, Johansson SK, Aspan A. Real‐time PCR for detection and differentiation of Streptococcus equi subsp. equi and Streptococcus equi subsp. zooepidemicus . Vet Microbiol. 2007;124:219‐229. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

