MECHANISMS IN ENDOCRINOLOGY: Human brown adipose tissue as a therapeutic target: warming up or cooling down?
- PMID: 33729178
- PMCID: PMC8111330
- DOI: 10.1530/EJE-20-1439
MECHANISMS IN ENDOCRINOLOGY: Human brown adipose tissue as a therapeutic target: warming up or cooling down?
Abstract
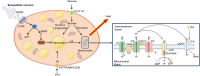
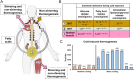
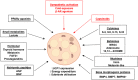
Excessive accumulation of white adipose tissue leads to obesity and its associated metabolic health consequences such as type 2 diabetes and cardiovascular disease. Several approaches to treat or prevent obesity including public health interventions, surgical weight loss, and pharmacological approaches to reduce caloric intake have failed to substantially modify the increasing prevalence of obesity. The (re-)discovery of active brown adipose tissue (BAT) in adult humans approximately 15 years ago led to a resurgence in research into whether BAT activation could be a novel therapy for the treatment of obesity. Upon cold stimulus, BAT activates and generates heat to maintain body temperature, thus increasing energy expenditure. Activation of BAT may provide a unique opportunity to increase energy expenditure without the need for exercise. However, much of the underlying mechanisms surrounding BAT activation are still being elucidated and the effectiveness of BAT as a therapeutic target has not been realised. Research is ongoing to determine how best to expand BAT mass and activate existing BAT; approaches include cold exposure, pharmacological stimulation using sympathomimetics, browning agents that induce formation of thermogenic beige adipocytes in white adipose depots, and the identification of factors secreted by BAT with therapeutic potential. In this review, we discuss the caloric capacity and other metabolic benefits from BAT activation in humans and the role of metabolic tissues such as skeletal muscle in increasing energy expenditure. We discuss the potential of current approaches and the challenges of BAT activation as a novel strategy to treat obesity and metabolic disorders.
Figures

References
-
- Who.int. Obesity and overweight [Web Page]. Internet (www.who.int): World Health Organizatuon, 2020. [updated 1 April 2020]. (available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight)
-
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017. 390 2627–2642. ( 10.1016/S0140-6736(17)32129-3) - DOI - PMC - PubMed
-
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF.et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014. 384 766–781. ( 10.1016/S0140-6736(14)60460-8) - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

