Choroidal and Retinal Changes After Systemic Adrenaline and Photodynamic Therapy in Non-Human Primates
- PMID: 33729474
- PMCID: PMC7980042
- DOI: 10.1167/iovs.62.3.25
Choroidal and Retinal Changes After Systemic Adrenaline and Photodynamic Therapy in Non-Human Primates
Abstract
Purpose: To determine the tomographic, angiographic, and histologic changes in the choroid and retina of cynomolgus monkeys after systemic adrenaline and verteporfin photodynamic therapy (vPDT).
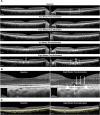
Methods: Six cynomolgus monkeys (12 eyes) were treated with vPDT only (n = 2), adrenaline only for eight weeks (n = 2), adrenaline for eight weeks with vPDT at week 4 (n = 4), and adrenaline for 12 weeks and vPDT at week 8 (n = 4). Spectral-domain optical coherence tomography, angiography, and autofluorescence were performed at baseline and every 14 days thereafter until 28 days after adrenaline therapy or vPDT. Choroid parameters included choroidal thickness (CT) changes and structural changes using semiautomated image binarization. Histology with light and electron microscopy was performed.
Results: Adrenaline resulted in subfoveal CT increase at week 4 compared with baseline (3.4%, P = 0.010), with further increase at week 8 (3.9%, P = 0.007). This correlated with choroidal luminal area increase (16.0% at week 8 compared with baseline, P = 0.030). Outer retinal changes included subretinal fluid, ellipsoid zone (EZ) disruption, photoreceptor elongation, and sub/intraretinal bright dots. Hypocyanescent spots surrounded by leakage was observed. Histology showed dilated choroidal vessels, intracytoplasmic vacuoles, and retinal pigment epithelium (RPE) enlarged basal infoldings. The vPDT decreased subfoveal CT at four weeks after vPDT (-7.5%, P = 0.007). This correlated with choroidal stromal area decrease (-18.0%, P < 0.010). Within the treatment spot, there was outer retinal atrophy, EZ disruption, irregular RPE thickening, intense hypoautofluorescence, hyperfluorescence, and hypocyanescence. On histology, there were outer retina, RPE, and choroid changes.
Conclusions: Adrenaline induces choroidal vessel dilation and CT increase. The vPDT decreases CT because of a reduction in choroidal stromal component.
Conflict of interest statement
Disclosure:
Figures






References
-
- Romano MR, Parolini B, Allegrini D, Mickalewska Z, Adelman R, Bonovas S, Bopp S, EVRS study group. An international collaborative evaluation of central serous chorioretinopathy: different therapeutic approaches and review of literature. The European Vitreoretinal Society central serous chorioretinopathy study [published online ahead of print December 6, 2019]. Acta Ophthalmol, 10.1111/aos.14319. - DOI - PubMed
-
- Kitzmann AS, Pulido JS, Diehl NN, Hodge DO, Burke JP.. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980-2002. Ophthalmology. 2008; 115: 169–173. - PubMed
-
- Spaide RF, Campeas L, Haas A, et al.. Central serous chorioretinopathy in younger and older adults. Ophthalmology. 1996; 103: 2070–2080. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

