Opioids for newborn infants receiving mechanical ventilation
- PMID: 33729556
- PMCID: PMC8121090
- DOI: 10.1002/14651858.CD013732.pub2
Opioids for newborn infants receiving mechanical ventilation
Abstract
Background: Mechanical ventilation is a potentially painful and discomforting intervention that is widely used in neonatal intensive care. Newborn infants demonstrate increased sensitivity to pain, which may affect clinical and neurodevelopmental outcomes. The use of drugs that reduce pain might be important in improving survival and neurodevelopmental outcomes.
Objectives: To determine the benefits and harms of opioid analgesics for neonates (term or preterm) receiving mechanical ventilation compared to placebo or no drug, other opioids, or other analgesics or sedatives.
Search methods: We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 9), in the Cochrane Library; MEDLINE via PubMed (1966 to 29 September 2020); Embase (1980 to 29 September 2020); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to 29 September 2020). We searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials and quasi-randomised trials.
Selection criteria: We included randomised and quasi-randomised controlled trials comparing opioids to placebo or no drug, to other opioids, or to other analgesics or sedatives in newborn infants on mechanical ventilation. We excluded cross-over trials. We included term (≥ 37 weeks' gestational age) and preterm (< 37 weeks' gestational age) newborn infants on mechanical ventilation. We included any duration of drug treatment and any dosage given continuously or as bolus; we excluded studies that gave opioids to ventilated infants for procedures.
Data collection and analysis: For each of the included trials, we independently extracted data (e.g. number of participants, birth weight, gestational age, types of opioids) using Cochrane Effective Practice and Organisation of Care Group (EPOC) criteria and assessed the risk of bias (e.g. adequacy of randomisation, blinding, completeness of follow-up). We evaluated treatment effects using a fixed-effect model with risk ratio (RR) for categorical data and mean difference (MD) for continuous data. We used the GRADE approach to assess the certainty of evidence.
Main results: We included 23 studies (enrolling 2023 infants) published between 1992 and 2019. Fifteen studies (1632 infants) compared the use of morphine or fentanyl versus placebo or no intervention. Four studies included both term and preterm infants, and one study only term infants; all other studies included only preterm infants, with five studies including only very preterm infants. We are uncertain whether opioids have an effect on the Premature Infant Pain Profile (PIPP) Scale in the first 12 hours after infusion (MD -5.74, 95% confidence interval (CI) -6.88 to -4.59; 50 participants, 2 studies) and between 12 and 48 hours after infusion (MD -0.98, 95% CI -1.35 to -0.61; 963 participants, 3 studies) because of limitations in study design, high heterogeneity (inconsistency), and imprecision of estimates (very low-certainty evidence - GRADE). The use of morphine or fentanyl probably has little or no effect in reducing duration of mechanical ventilation (MD 0.23 days, 95% CI -0.38 to 0.83; 1259 participants, 7 studies; moderate-certainty evidence because of unclear risk of bias in most studies) and neonatal mortality (RR 1.12, 95% CI 0.80 to 1.55; 1189 participants, 5 studies; moderate-certainty evidence because of imprecision of estimates). We are uncertain whether opioids have an effect on neurodevelopmental outcomes at 18 to 24 months (RR 2.00, 95% CI 0.39 to 10.29; 78 participants, 1 study; very low-certainty evidence because of serious imprecision of the estimates and indirectness). Limited data were available for the other comparisons (i.e. two studies (54 infants) on morphine versus midazolam, three (222 infants) on morphine versus fentanyl, and one each on morphine versus diamorphine (88 infants), morphine versus remifentanil (20 infants), fentanyl versus sufentanil (20 infants), and fentanyl versus remifentanil (24 infants)). For these comparisons, no meta-analysis was conducted because outcomes were reported by one study.
Authors' conclusions: We are uncertain whether opioids have an effect on pain and neurodevelopmental outcomes at 18 to 24 months; the use of morphine or fentanyl probably has little or no effect in reducing the duration of mechanical ventilation and neonatal mortality. Data on the other comparisons planned in this review (opioids versus analgesics; opioids versus other opioids) are extremely limited and do not allow any conclusions. In the absence of firm evidence to support a routine policy, opioids should be used selectively - based on clinical judgement and evaluation of pain indicators - although pain measurement in newborns has limitations.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
RB has no interest to declare.
KAdW has no interest to declare.
RZ has no interest to declare.
OR has no interest to declare.
CN has no interest to declare.
MB has received research funding from an ALF grant (non‐profit ‐ Lund University) and from the Crafoord Foundation (non‐profit) for research projects not related to Cochrane.
Figures
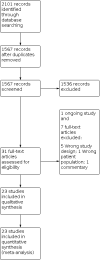










































Update of
References
References to studies included in this review
Anand 1999 {published data only}
-
- Anand KJ, Barton BA, McIntosh N, Lagercrantz H, Pelausa E, Young TE, et al. Analgesia and sedation in preterm neonates who require ventilatory support: results from the NOPAIN trial. Neonatal Outcome and Prolonged Analgesia in Neonates. Archives of Pediatrics & Adolescent Medicine 1999;153(4):331-8. [10.1001/archpedi.153.4.331] [PMID: ] - PubMed
-
- Anand KJ, McIntosh N, Lagercrantz H, Gauthier T, Pelausa E, Young T, et al. The Pilot NOPAIN Trial: morphine and midazolam infusions decrease pain/stress and may alter clinical outcomes in ventilated preterm neonates. Pediatric Research 1997;41(4 Pt 2):136A. [PMID: 10.1203/00006450-199704001-00819] - DOI
-
- Anand KJS, McIntosh N, Lagercrantz H, Young TE, Vasa R, Barton BA. Erratum: Analgesia and sedation in preterm neonates who require ventilatory support: result from the NOPAIN trial (Archives of Pediatrics and Adolescent Medicine (April 1999) 153 (331-338)). Archives of Pediatrics and Adolescent Medicine 1999;153(8):895. - PubMed
Anand 2004 {published data only}
-
- Anand KJ, Hall RW, Desai N, Shephard B, Bergqvist LL, Young TE, et al, NEOPAIN Trial Investigators Group. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet 2004;363(9422):1673-82. [PMID: 10.1016/S0140-6736(04)16251-X] [PMID: ] - DOI - PubMed
-
- Boyle EM, Wong CM, Freer Y, Ahmadian M, Maxwell A, McIntosh N, et al. Mean heart rate and heart rate variability in preterm ventilated infants receiving morphine analgesia. Pediatric Research 2003;54:569.
-
- Ferguson SA, Ward WL, Paule MG, Hall RW, Anand KJ. A pilot study of preemptive morphine analgesia in preterm neonates: effects on head circumference, social behavior, and response latencies in early childhood. Neurotoxicology and Teratology 2012;34(1):47-55. [PMID: 10.1016/j.ntt.2011.10.008] [PMID: ] - DOI - PubMed
Ancora 2013 {published data only}
-
- Ancora G, Lago P, Garetti E, Pirelli A, Merazzi D, Mastrocola M, et al. Efficacy and safety of continuous infusion of fentanyl for pain control in preterm newborns on mechanical ventilation (MV). In: Pediatric Academic Societies (PAS) Annual Meeting. 2011. - PubMed
-
- Ancora G, Lago P, Garetti E, Pirelli A, Merazzi D, Pierantoni L, et al. Follow-up at the corrected age of 24 months of preterm newborns receiving continuous infusion of fentanyl for pain control during mechanical ventilation. Pain 2017;158(5):840-5. [DOI: 10.1097/j.pain.0000000000000839] [PMID: ] - DOI - PubMed
-
- Ancora G, Pierantoni L, Grandi S, Maranella E. Continuous infusion of fentanyl in preterm on mechanical ventilation. In: 50th Annual Meeting of the European Society for Paediatric Research. 2009.
-
- NCT00571636. Continuous infusion of fentanyl in preterm on MV. clinicaltrials.gov/ct2/show/study/NCT00571636 (first received 9 May 2006).
Chen 2015 {published data only}
-
- Chen SS, Liu L, Hu P, Shi BZ, Fu YK, Luo R, et al. [Analgesic effect of fentanyl in neonates during mechanical ventilation]. Zhongguo dang dai er ke za zhi = Chinese Journal of Contemporary Pediatrics 2015;17(10):1045-50. [PMID: ] - PubMed
Dyke 1995 {published data only}
e Silva 2008 {published data only}
Guinsburg 1998 {published data only}
Ionides 1994 {published data only}
Jiang 2012 {published data only}
-
- Jiang HH, Cheng R, Kan Q, Shen X, Li F, Fu CH, et al. [Clinical evaluation of the effects of morphine in mechanical ventilation of neonates]. Zhonghua er ke za zhi = Chinese Journal of Pediatrics 2012;50(5):350-5. [PMID: ] - PubMed
Lago 1998 {published data only}
Lago 1999 {published data only}
-
- Lago P, Benini F, Salvadori S, Bettiol T, Agosto C, Zacchello F. Effect of administering low-dose fentanyl infusion on respiratory dynamics in the premature ventilated for respiratory distress syndrome - a randomized double-blind trial. Pediatric Research 1999;45(7):308. [DOI: 10.1203/00006450-199904020-01835] - DOI
Liem 1999 {published data only}
-
- Liem KD, Der Velden AA, Klaessens JH, De Bor M. Changes of cerebral oxygenation and hemodynamics in ventilated preterm infants after sedation with midazolam and morphine: preliminary report. Pediatric Research 1999;45(6):906. [DOI: 10.1203/00006450-199906000-00134] - DOI
Naderi 2017 {published data only}
-
- Naderi S, Goodarzi R, Naziri GRP, Mohammad AM, Kheiltash A, Shafaeizadeh A. Effect of fentanyl and morphine on gallbladder dimensions in newborns admitted to the neonatal intensive care unit: a randomized double-blinded clinical trial. Iranian Journal of Pediatrics 2017;27(1):e5726. [DOI: 10.5812/ijp.5726] - DOI
Orsini 1996 {published data only}
Qiu 2019 {published data only}
Quinn 1992 {published data only}
Quinn 1993 {published data only}
Saarenmaa 1999 {published data only}
Schmidt 2010 {published data only}
Simons 2003 {published data only}
-
- Graaf J, den Akker EL, Lingen RA, Groot Jebbink LJ, Jong FH, Grunau RE, et al. Five-year follow-up of effects of neonatal intensive care and morphine infusion during mechanical ventilation on diurnal cortisol rhythm. Journal of Pediatrics 2014;165(3):459-63.e2. [PMID: ] - PubMed
-
- Graaf J, Lingen RA, Simons SH, Anand KJ, Duivenvoorden HJ, Weisglas-Kuperus N, et al. Long-term effects of routine morphine infusion in mechanically ventilated neonates on children's functioning: five-year follow-up of a randomized controlled trial. Pain 2011;152(6):1391-7. [PMID: ] - PubMed
-
- Graaf J, Lingen RA, Valkenburg AJ, Weisglas-Kuperus N, Groot Jebbink L, Wijnberg-Williams B, et al. Does neonatal morphine use affect neuropsychological outcomes at 8 to 9 years of age? Pain 2013;154(3):449-58. [PMID: ] - PubMed
-
- Simons SH, Dijk M, Lingen RA, Roofthooft D, Boomsma F, den Anker JN, et al. Randomised controlled trial evaluating effects of morphine on plasma adrenaline/noradrenaline concentrations in newborns. Archives of Disease in Childhood. Fetal and Neonatal Edition 2005;90(1):F36-40. [PMID: ] - PMC - PubMed
Siwiec 1999 {published data only}
-
- Siwiec J, Porzucek I, Gadzinowski J, Bhat R, Vidyasagar D. Effect of short term morphine infusion on premature infant pain profile (PIPP) and hemodynamics. Pediatric Research 1999;45(7):69. [DOI: 10.1203/00006450-199904020-00416] - DOI
Welzing 2012 {published data only}
-
- NCT00419601. RAPIP study: clinical trial on remifentanil for analgesia and sedation of ventilated neonates and infants. clinicaltrials.gov/ct2/show/NCT00419601 (first received 8 January 2007).
-
- Welzing L, Link F, Junghaenel S, Oberthuer A, Harnischmacher U, Stuetzer H, et al. Remifentanil-induced tolerance, withdrawal or hyperalgesia in infants: a randomized controlled trial. RAPIP trial: remifentanil-based analgesia and sedation of paediatric intensive care patients. Neonatology 2013;104(1):34-41. [DOI: 10.1159/000348790] [PMID: ] - DOI - PubMed
-
- Welzing L, Oberthuer A, Junghaenel S, Harnischmacher U, Stutzer H, Roth B. Remifentanil/midazolam versus fentanyl/midazolam for analgesia and sedation of mechanically ventilated neonates and young infants: a randomized controlled trial. Intensive Care Medicine 2012;38(6):1017-24. [DOI: 10.1007/s00134-012-2532-1] [PMID: ] - DOI - PubMed
Wood 1998 {published data only}
References to studies excluded from this review
Akkermans 2015 {published data only}
Anand 2008 {published data only}
Bell 1987 {published data only}
-
- Bell SG, Ellis LJ. Use of fentanyl for sedation of mechanically ventilated neonates. Neonatal Network 1987;6(2):27-31. [PMID: ] - PubMed
Bergqvist 2007 {published data only}
-
- Bergqvist L, Eriksson M, Kronsberg S, Schollin J, Barton B, Anand K. Seeing through the blind! Ability of hospital staff to differentiate morphine from placebo, in neonates at a placebo controlled trial. Acta Paediatrica 2007;96(7):1004-7. [DOI: 10.1111/j.1651-2227.2007.00270.x] [PMID: ] - DOI - PubMed
Hwang 1999 {published data only}
-
- Hwang WK, Kim HM. Pain reduction effects of continuous fentanyl infusion to intensive care neonates. Journal of the Korean Pediatric Society 1999;42(5):657-65.
Palmer 2005 {published data only}
References to ongoing studies
IRCT2017082417413N26 {published data only}
-
- IRCT2017082417413N26. Comparing the effect of fentanyl and midazolam on the sedation of infants under mechanical ventilation: a randomized clinical trial. en.irct.ir/trial/16036 (first received 2016 July 1).
Additional references
AAP 2016
Anand 1987
Anand 1990
Anand 1993
Anand 1998
Anand 2001
-
- Anand KJ, International Evidence-Based Group for Neonatal Pain. Consensus statement for the prevention and management of pain in the newborn. Archives of Pediatric and Adolescent Medicine 2001;155(2):173-80. [PMID: ] - PubMed
Anand 2013
-
- Anand KJ, Clark AE, Willson DF, Berger J, Meert KL, Zimmerman JJ, et al, Eunic Kennedy Shriver National Institute of Child Health, Human Development (NICHD) Collaborative Pediatric Critical Care Research Network (CPCCRN). Opioid analgesia in mechanically ventilated children: results from the multicenter Measuring Opioid Tolerance Induced by Fentanyl study. Pediatric Critical Care Medicine 2013;14(1):27-36. [DOI: 10.1097/PCC.0b013e318253c80e] [PMID: ] - DOI - PMC - PubMed
Anand 2017
-
- Anand KJ, Eriksson M, Boyle EM, Avila-Alvarez A, Andersen RD, Sarafidis K, et al, EUROPAIN survey working group of the NeoOpioid Consortium. Assessment of continuous pain in newborns admitted to NICUs in 18 European countries. Acta Paediatrica 2017;106(8):1248-59. [DOI: 10.1111/apa.13810] [PMID: ] - DOI - PubMed
Ancora 2017
-
- Ancora G, Lago P, Garetti E, Pirelli A, Merazzi D, Pierantoni L, et al. Follow-up at the corrected age of 24 months of preterm newborns receiving continuous infusion of fentanyl for pain control during mechanical ventilation. Pain 2017;158(5):840-5. [DOI: 10.1097/j.pain.0000000000000839] [PMID: ] - DOI - PubMed
Ancora 2019
-
- Ancora G, Lago P, Garetti E, Merazzi D, Savant Levet P, Bellieni CV, et al. Evidence-based clinical guidelines on analgesia and sedation in newborn infants undergoing assisted ventilation and endotracheal intubation. Acta Paediatrica 2019;108(2):208-17. [DOI: 10.1111/apa.14606] [PMID: ] - PubMed
Aranda 2005
Ballantyne 1999
Barker 1996
Bayley 1993
-
- Bayley N. Bayley Scales of Infant Development. 2nd edition. San Antonio (TX): The Psychological Corporation, 1993.
Bayley 2006
-
- Bayley N. Bayley Scales of Infant and Toddler Development. San Antonio (TX): Harcourt Assessment, 2006.
Bell 1978
Carbajal 2015
-
- Carbajal R, Eriksson M, Courtois E, Boyle E, Avila-Alvarez A, Andersen DR, et al, EUROPAIN Survey Working Group. Sedation and analgesia practices in neonatal intensive care units (EUROPAIN): results from a prospective cohort study. The Lancet Respiratory Medicine 2015;3(10):796-812. [DOI: 10.1016/S2213-2600(15)00331-8] [PMID: ] - DOI - PubMed
Cochrane EPOC 2017
-
- Cochrane Effective Practice and Organisation of Care (EPOC). EPOC resources for review authors, 2017. epoc.cochrane.org/epoc-resources-review-authors (accessed prior to 12 July 2019).
Darnall 2010
Debillon 2001
-
- Debillon T, Zupan V, Ravault N, Magny JF, Dehan M. Development and initial validation of the EDIN scale, a new tool for assessing prolonged pain in preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2001;85(1):F36-41. [DOI: 10.1136/fn.85.1.f36] [PMID: ] - DOI - PMC - PubMed
de Graaf 2011
-
- Graaf J, Lingen RA, Simons SH, Anand KJ, Duivenvoorden HJ, Weisglas-Kuperus N, et al. Long-term effects of routine morphine infusion in mechanically ventilated neonates on children's functioning: five-year follow-up of a randomized controlled trial. Pain 2011;152(6):1391-7. [PMID: ] - PubMed
de Graaf 2013
-
- Graaf J, Lingen RA, Valkenburg AJ, Weisglas-Kuperus N, Groot Jebbink L, Wijnberg-Williams B, et al. Does neonatal morphine use affect neuropsychological outcomes at 8 to 9 years of age? Pain 2013;154(3):449-58. [PMID: ] - PubMed
de Graaf 2014
-
- Graaf J, den Akker EL, Lingen RA, Groot Jebbink LJ, Jong FH, Grunau RE, et al. Five-year follow-up of effects of neonatal intensive care and morphine infusion during mechanical ventilation on diurnal cortisol rhythm. Journal of Pediatrics 2014;165(3):459-63.e2. [PMID: ] - PubMed
de Vries 1992
Fink 2015
Fitzgerald 1989
Giordano 2019
-
- Giordano V, Edobor J, Deindl P, Wildner B, Goeral K, Steinbauer P, et al. Pain and sedation scales for neonatal and pediatric patients in a preverbal stage of development: a systematic review. JAMA Pediatrics 2019;173(12):1186-97. [DOI: 173 10.1001/jamapediatrics.2019.3351] [PMID: ] - PubMed
Golianu 2007
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 3 May 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Griffiths 1954
-
- Griffiths R. The Abilities of Babies: A Study in Mental Measurement. New York (NY): McGraw-Hill Book Co. Inc, 1954.
Griffiths 1970
-
- Griffiths R. The Abilities of Young Children: A Comprehensive System of Mental Measurement for the First Eight Years. London (UK): Child Development Research Centre, 1970.
Grunau 1990
-
- Grunau RV, Craig KD. Facial activity as a measure of neonatal pain expression. In: Tyler DC, Krane EJ, editors(s). Advances in Pain Research Therapy. Pediatric Pain. Vol. 15. New York (NY): Raven Press, 1990:147-54.
Hall 2007
Higgins 2003
Higgins 2011a
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JP, Altman DG, Sterne JA, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8. Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hummel 2008
-
- Hummel P, Puchalski M, Creech SD, Weiss MG. Clinical reliability and validity of the N-PASS: neonatal pain, agitation and sedation scale with prolonged pain. Journal of Perinatology 2008;28(1):55-60. [PMID: ] - PubMed
ICCROP 2005
Jacobs 2013
Jobe 2001
Kahn 1998
Kinoshita 2020
Krechel 1995
Lago 2017
Larsson 1999
Lawrence 1993
-
- Lawrence J, Alcock D, McGrath P, Kay J, MacMurray SB, Dulberg C. The development of a tool to assess neonatal pain. Neonatal Network 1993;12(6):59-66. [PMID: ] - PubMed
Lundqvist 2014
MacGregor 1998
Menon 1998
Moher 2009
Nemergut 2013
Ng 2017
NIH 1979
-
- National Institutes of Heart, Lung, and Blood Institute of Lung Diseases. In: Report of Workshop on Bronchopulmonary Dysplasia; Dec 4-8 1978. Bethesda (MD): U.S. Dept. of Health, Education, and Welfare, Public Health Service, National Institutes of Health, 1979.
Olsson 2021
Papile 1978
Pillai 2015
Purcell‐Jones 1988
RevMan Web [Computer program]
-
- The Cochrane Collaboration Review Manager Web (RevMan Web). The Cochrane Collaboration, 2019. Available at revman.cochrane.org.
Romantsik 2017a
Romantsik 2017b
-
- Romantsik O, Bruschettini M, Calevo MG, Banzi R, Ley D. Pharmacological pain and sedation interventions for the prevention of intraventricular haemorrhage in preterm infants on assisted ventilation - an overview of systematic reviews. Cochrane Database of Systematic Reviews 2017, Issue 6. Art. No: CD012706. [DOI: 10.1002/14651858.CD012706] - DOI - PMC - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Simons 2005
-
- Simons SH, Dijk M, Lingen RA, Roofthooft D, Boomsma F, den Anker JN, et al. Randomised controlled trial evaluating effects of morphine on plasma adrenaline/noradrenaline concentrations in newborns. Archives of Disease in Childhood. Fetal and Neonatal Edition 2005;90(1):F36-40. [PMID: ] - PMC - PubMed
Soll 2013
Stevens 1996
Taddio 2002
Taddio 2009
Valkenburg 2015
-
- Valkenburg AJ, den Bosch GE, Graaf J, Lingen RA, Weisglas-Kuperus N, Rosmalen J, et al. Long-term effects of neonatal morphine infusion on pain sensitivity: follow-up of a randomized controlled trial. Journal of Pain 2015;16(9):926-33. [PMID: ] - PubMed
van Dijk 2009
Vendettuoli 2014
Walsh 2004
-
- Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, et al, National Institute of Child Health and Human Development Neonatal Research Network. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 2004;114(5):1305-11. [DOI: 10.1542/peds.2004-0204 ] [PMID: ] - PubMed
References to other published versions of this review
Bellù 2005
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

