Bioinspired Diversification Approach Toward the Total Synthesis of Lycodine-Type Alkaloids
- PMID: 33729783
- PMCID: PMC8017526
- DOI: 10.1021/jacs.1c00457
Bioinspired Diversification Approach Toward the Total Synthesis of Lycodine-Type Alkaloids
Abstract
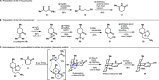
Nitrogen heterocycles (azacycles) are common structural motifs in numerous pharmaceuticals, agrochemicals, and natural products. Many powerful methods have been developed and continue to be advanced for the selective installation and modification of nitrogen heterocycles through C-H functionalization and C-C cleavage approaches, revealing new strategies for the synthesis of targets containing these structural entities. Here, we report the first total syntheses of the lycodine-type Lycopodium alkaloids casuarinine H, lycoplatyrine B, lycoplatyrine A, and lycopladine F as well as the total synthesis of 8,15-dihydrohuperzine A through bioinspired late-stage diversification of a readily accessible common precursor, N-desmethyl-β-obscurine. Key steps in the syntheses include oxidative C-C bond cleavage of a piperidine ring in the core structure of the obscurine intermediate and site-selective C-H borylation of a pyridine nucleus to enable cross-coupling reactions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Siengalewicz P.; Mulzer J.; Rinner U.. Chapter 1: Lycopodium Alkaloids—Synthetic Highlights and Recent Developments. In The Alkaloids: Chemistry and Biology; Knölker H.-J., Ed.; Academic Press: Cambridge, MA, 2013; Vol. 72, pp 1–151. - PubMed
-
- Bödeker K. Lycopodin, das erste Alkaloïd der Gefässkryptogamen. Liebigs Ann. 1881, 208, 363–367. 10.1002/jlac.18812080308. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

