AIRE deficiency, from preclinical models to human APECED disease
- PMID: 33729987
- PMCID: PMC7875492
- DOI: 10.1242/dmm.046359
AIRE deficiency, from preclinical models to human APECED disease
Abstract
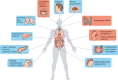
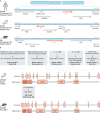
Autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED) is a rare life-threatening autoimmune disease that attacks multiple organs and has its onset in childhood. It is an inherited condition caused by a variety of mutations in the autoimmune regulator (AIRE) gene that encodes a protein whose function has been uncovered by the generation and study of Aire-KO mice. These provided invaluable insights into the link between AIRE expression in medullary thymic epithelial cells (mTECs), and the broad spectrum of self-antigens that these cells express and present to the developing thymocytes. However, these murine models poorly recapitulate all phenotypic aspects of human APECED. Unlike Aire-KO mice, the recently generated Aire-KO rat model presents visual features, organ lymphocytic infiltrations and production of autoantibodies that resemble those observed in APECED patients, making the rat model a main research asset. In addition, ex vivo models of AIRE-dependent self-antigen expression in primary mTECs have been successfully set up. Thymus organoids based on pluripotent stem cell-derived TECs from APECED patients are also emerging, and constitute a promising tool to engineer AIRE-corrected mTECs and restore the generation of regulatory T cells. Eventually, these new models will undoubtedly lead to main advances in the identification and assessment of specific and efficient new therapeutic strategies aiming to restore immunological tolerance in APECED patients.
Keywords: AIRE; APECED; APS-1; Knockout model; Organoid; mTEC.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


References
-
- Akiyama, T., Shimo, Y., Yanai, H., Qin, J., Ohshima, D., Maruyama, Y., Asaumi, Y., Kitazawa, J., Takayanagi, H., Penninger, J. M.et al. (2008). The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity 29, 423-437. 10.1016/j.immuni.2008.06.015 - DOI - PubMed
-
- Anderson, M. S., Venanzi, E. S., Klein, L., Chen, Z., Berzins, S. P., Turley, S. J., von Boehmer, H., Bronson, R., Dierich, A., Benoist, Cet al. et al. (2002). Projection of an immunological self shadow within the thymus by the aire protein. Science 298, 1395-1401. 10.1126/science.1075958 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

