The microtubule-associated protein WDL4 modulates auxin distribution to promote apical hook opening in Arabidopsis
- PMID: 33730147
- PMCID: PMC8290285
- DOI: 10.1093/plcell/koab080
The microtubule-associated protein WDL4 modulates auxin distribution to promote apical hook opening in Arabidopsis
Abstract
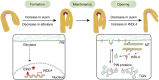
The unique apical hook in dicotyledonous plants protects the shoot apical meristem and cotyledons when seedlings emerge through the soil. Its formation involves differential cell growth under the coordinated control of plant hormones, especially ethylene and auxin. Microtubules are essential players in plant cell growth that are regulated by multiple microtubule-associated proteins (MAPs). However, the role and underlying mechanisms of MAP-microtubule modules in differential cell growth are poorly understood. In this study, we found that the previously uncharacterized Arabidopsis MAP WAVE-DAMPENED2-LIKE4 (WDL4) protein plays a positive role in apical hook opening. WDL4 exhibits a temporal expression pattern during hook development in dark-grown seedlings that is directly regulated by ethylene signaling. WDL4 mutants showed a delayed hook opening phenotype while overexpression of WDL4 resulted in enhanced hook opening. In particular, wdl4-1 mutants exhibited stronger auxin accumulation in the concave side of the apical hook. Furthermore, the regulation of the auxin maxima and trafficking of the auxin efflux carriers PIN-FORMED1 (PIN1) and PIN7 in the hook region is critical for WDL4-mediated hook opening. Together, our study demonstrates that WDL4 positively regulates apical hook opening by modulating auxin distribution, thus unraveling a mechanism for MAP-mediated differential plant cell growth.
© American Society of Plant Biologists 2021. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures







Similar articles
-
WAG2 represses apical hook opening downstream from gibberellin and PHYTOCHROME INTERACTING FACTOR 5.Development. 2012 Nov;139(21):4020-8. doi: 10.1242/dev.081240. Epub 2012 Sep 19. Development. 2012. PMID: 22992959
-
The auxin influx carriers AUX1 and LAX3 are involved in auxin-ethylene interactions during apical hook development in Arabidopsis thaliana seedlings.Development. 2010 Feb;137(4):597-606. doi: 10.1242/dev.040790. Development. 2010. PMID: 20110325
-
Role of PIN-mediated auxin efflux in apical hook development of Arabidopsis thaliana.Development. 2010 Feb;137(4):607-17. doi: 10.1242/dev.041277. Development. 2010. PMID: 20110326
-
[The molecular mechanism of apical hook development in dicot plant].Yi Chuan. 2021 Aug 20;43(8):723-736. doi: 10.16288/j.yczz.21-105. Yi Chuan. 2021. PMID: 34413013 Review. Chinese.
-
Regulation of seedling growth by ethylene and the ethylene-auxin crosstalk.Planta. 2017 Mar;245(3):467-489. doi: 10.1007/s00425-017-2651-6. Epub 2017 Feb 10. Planta. 2017. PMID: 28188422 Review.
Cited by
-
SUPPRESSOR OF PHYTOCHROME B-4 #3 reduces the expression of PIF-activated genes and increases expression of growth repressors to regulate hypocotyl elongation in short days.BMC Plant Biol. 2022 Aug 15;22(1):399. doi: 10.1186/s12870-022-03737-z. BMC Plant Biol. 2022. PMID: 35965321 Free PMC article.
-
HY5 inhibits lateral root initiation in Arabidopsis through negative regulation of the microtubule-stabilizing protein TPXL5.Plant Cell. 2023 Mar 15;35(3):1092-1109. doi: 10.1093/plcell/koac358. Plant Cell. 2023. PMID: 36512471 Free PMC article.
-
GR24, A Synthetic Strigolactone Analog, and Light Affect the Organization of Cortical Microtubules in Arabidopsis Hypocotyl Cells.Front Plant Sci. 2021 Jul 7;12:675981. doi: 10.3389/fpls.2021.675981. eCollection 2021. Front Plant Sci. 2021. PMID: 34305975 Free PMC article.
-
Microtubule Regulation in Plants: From Morphological Development to Stress Adaptation.Biomolecules. 2023 Mar 30;13(4):627. doi: 10.3390/biom13040627. Biomolecules. 2023. PMID: 37189374 Free PMC article. Review.
-
Ethylene Signaling Facilitates Plant Adaption to Physical Barriers.Front Plant Sci. 2021 Jul 29;12:697988. doi: 10.3389/fpls.2021.697988. eCollection 2021. Front Plant Sci. 2021. PMID: 34394151 Free PMC article. Review.
References
-
- Abas L, Benjamins R, Malenica N, Paciorek T, Wisniewska J, Moulinier-Anzola JC, Sieberer T, Friml J, Luschnig C (2006) Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat Cell Biol 8: 249–256 - PubMed
-
- Ambrose C, Ruan Y, Gardiner J, Tamblyn LM, Catching A, Kirik V, Marc J, Overall R, Wasteneys GO (2013) CLASP interacts with sorting nexin 1 to link microtubules and auxin transport via PIN2 recycling in Arabidopsis thaliana. Dev Cell 24: 649–659 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

