The Metallocene Battery: Ultrafast Electron Transfer Self Exchange Rate Accompanied by a Harmonic Height Breathing
- PMID: 33730408
- PMCID: PMC8252062
- DOI: 10.1002/anie.202100174
The Metallocene Battery: Ultrafast Electron Transfer Self Exchange Rate Accompanied by a Harmonic Height Breathing
Abstract
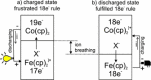
The first all-metallocene rechargeable battery consisting of poly-cobaltocenium/- and poly-ferrocene/reduced graphene oxide composites as anode and cathode was prepared. The intrinsically fast ET self-exchange rate of metallocenes was successfully combined with an efficient ion-percolation achieved by molecular self-assembly. The resulting battery materials show ideal Nernstian behavior, is thickness scalable up to >1.2 C cm-2 , and exhibit high coulombic efficiency at ultrafast rates (200 A g-1 ). Using aqueous LiClO4 , the charge is carried exclusively by the anion. The ClO4 - intercalation is accompanied by a reciprocal height change of the active layers. Principally, volume changes in organic battery materials during charging/discharging are not desirable and represent a major safety issue. However, here, the individual height changes-due to ion breathing-are reciprocal and thus prohibiting any internal pressure build-up in the closed-cell, leading to excellent cycling stability.
Keywords: cobaltocene; ferrocene; organic batteries; organometallic electrodes; reduced graphene oxide.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The Li-ion rechargeable battery: a perspective.J Am Chem Soc. 2013 Jan 30;135(4):1167-76. doi: 10.1021/ja3091438. Epub 2013 Jan 18. J Am Chem Soc. 2013. PMID: 23294028
-
The staging mechanism of AlCl4 intercalation in a graphite electrode for an aluminium-ion battery.Phys Chem Chem Phys. 2017 Mar 15;19(11):7980-7989. doi: 10.1039/c7cp00453b. Phys Chem Chem Phys. 2017. PMID: 28263339
-
Carbon Nanoscrolls for Aluminum Battery.ACS Nano. 2018 Aug 28;12(8):8456-8466. doi: 10.1021/acsnano.8b03961. Epub 2018 Jul 30. ACS Nano. 2018. PMID: 30048113
-
High Performance Poly(viologen)-Graphene Nanocomposite Battery Materials with Puff Paste Architecture.ACS Nano. 2017 Sep 26;11(9):8730-8740. doi: 10.1021/acsnano.7b02310. Epub 2017 Aug 28. ACS Nano. 2017. PMID: 28836762
-
Aqueous Rechargeable Zn-ion Batteries: Strategies for Improving the Energy Storage Performance.ChemSusChem. 2021 May 6;14(9):1987-2022. doi: 10.1002/cssc.202100299. Epub 2021 Apr 8. ChemSusChem. 2021. PMID: 33725419 Review.
Cited by
-
Wireless electrochemical light emission in ultrathin 2D nanoconfinements.Chem Sci. 2022 Nov 21;13(48):14277-14284. doi: 10.1039/d2sc04670a. eCollection 2022 Dec 14. Chem Sci. 2022. PMID: 36545138 Free PMC article.
-
When a Side Reaction Is a Benefit: A Catalyst-Free Route to Obtain High-Molecular Cobaltocenium-Functionalized Polysiloxanes by Hydroamination.Polymers (Basel). 2024 Oct 14;16(20):2887. doi: 10.3390/polym16202887. Polymers (Basel). 2024. PMID: 39458715 Free PMC article.
References
-
- Ding Y., Zhao Y., Li Y., Goodenough J. B., Yu G., Energy Environ. Sci. 2017, 10, 491–497.
-
- Hultgren V. M., Mariotti A. W. A., Bond A. M., Wedd A. G., Anal. Chem. 2002, 74, 3151–3156. - PubMed
-
- Frew J. E., Hill H. A. O., Eur. J. Biochem. 1988, 172, 261–269. - PubMed
-
- Astruc D., Ornelas C., Ruiz J., Chem. Eur. J. 2009, 15, 8936–8944. - PubMed
-
- Abraham K. M., Pasquariello D. M., Willstaedt E. B., J. Electrochem. Soc. 1990, 137, 1856–1857.
LinkOut - more resources
Full Text Sources
Other Literature Sources

