Defect Passivation in Lead-Halide Perovskite Nanocrystals and Thin Films: Toward Efficient LEDs and Solar Cells
- PMID: 33730428
- PMCID: PMC8518834
- DOI: 10.1002/anie.202102360
Defect Passivation in Lead-Halide Perovskite Nanocrystals and Thin Films: Toward Efficient LEDs and Solar Cells
Abstract
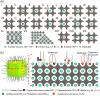
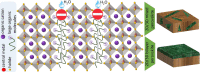
Lead-halide perovskites (LHPs), in the form of both colloidal nanocrystals (NCs) and thin films, have emerged over the past decade as leading candidates for next-generation, efficient light-emitting diodes (LEDs) and solar cells. Owing to their high photoluminescence quantum yields (PLQYs), LHPs efficiently convert injected charge carriers into light and vice versa. However, despite the defect-tolerance of LHPs, defects at the surface of colloidal NCs and grain boundaries in thin films play a critical role in charge-carrier transport and nonradiative recombination, which lowers the PLQYs, device efficiency, and stability. Therefore, understanding the defects that play a key role in limiting performance, and developing effective passivation routes are critical for achieving advances in performance. This Review presents the current understanding of defects in halide perovskites and their influence on the optical and charge-carrier transport properties. Passivation strategies toward improving the efficiencies of perovskite-based LEDs and solar cells are also discussed.
Keywords: defect passivation; lead-halide perovskites; perovskite nanocrystals; perovskite solar cells; surface chemistry.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- None
-
- Queisser H. J., Haller E. E., Science 1998, 281, 945–950; - PubMed
-
- McCluskey M. D., Janotti A., J. Appl. Phys. 2020, 127, 190401;
-
- Mahajan S., Acta Mater. 2000, 48, 137–149;
-
- Pantelides S. T., Rev. Mod. Phys. 1978, 50, 797–858;
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

