In Utero Exposure to Mercury Is Associated With Increased Susceptibility to Liver Injury and Inflammation in Childhood
- PMID: 33730435
- PMCID: PMC8446089
- DOI: 10.1002/hep.31809
In Utero Exposure to Mercury Is Associated With Increased Susceptibility to Liver Injury and Inflammation in Childhood
Abstract
Background and aims: Nonalcoholic fatty liver disease (NAFLD) is the most prevalent cause of liver disease in children. Mercury (Hg), a ubiquitous toxic metal, has been proposed as an environmental factor contributing to toxicant-associated fatty liver disease.
Approach and results: We investigated the effect of prenatal exposure to Hg on childhood liver injury by combining epidemiological results from a multicenter mother-child cohort with complementary in vitro experiments on monocyte cells that are known to play a key role in liver immune homeostasis and NAFLD. We used data from 872 mothers and their children (median age, 8.1 years; interquartile range [IQR], 6.5-8.7) from the European Human Early-Life Exposome cohort. We measured Hg concentration in maternal blood during pregnancy (median, 2.0 μg/L; IQR, 1.1-3.6). We also assessed serum levels of alanine aminotransferase (ALT), a common screening tool for pediatric NAFLD, and plasma concentrations of inflammation-related cytokines in children. We found that prenatal Hg exposure was associated with a phenotype in children that was characterized by elevated ALT (≥22.1 U/L for females and ≥25.8 U/L for males) and increased concentrations of circulating IL-1β, IL-6, IL-8, and TNF-α. Consistently, inflammatory monocytes exposed in vitro to a physiologically relevant dose of Hg demonstrated significant up-regulation of genes encoding these four cytokines and increased concentrations of IL-8 and TNF-α in the supernatants.
Conclusions: These findings suggest that developmental exposure to Hg can contribute to inflammation and increased NAFLD risk in early life.
© 2021 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
Figures


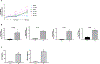
Comment in
-
Letter to the editor: In utero exposure to mercury is associated with increased susceptibility to liver injury and inflammation in childhood.Hepatology. 2023 Feb 1;77(2):E38. doi: 10.1002/hep.32768. Epub 2022 Sep 23. Hepatology. 2023. PMID: 36054063 No abstract available.
Similar articles
-
Prenatal Exposure to Perfluoroalkyl Substances Associated With Increased Susceptibility to Liver Injury in Children.Hepatology. 2020 Nov;72(5):1758-1770. doi: 10.1002/hep.31483. Epub 2020 Oct 19. Hepatology. 2020. PMID: 32738061 Free PMC article.
-
Associations between mercury exposure and the risk of nonalcoholic fatty liver disease (NAFLD) in US adolescents.Environ Sci Pollut Res Int. 2019 Oct;26(30):31384-31391. doi: 10.1007/s11356-019-06224-5. Epub 2019 Aug 31. Environ Sci Pollut Res Int. 2019. PMID: 31473923
-
Association of Fish Consumption and Mercury Exposure During Pregnancy With Metabolic Health and Inflammatory Biomarkers in Children.JAMA Netw Open. 2020 Mar 2;3(3):e201007. doi: 10.1001/jamanetworkopen.2020.1007. JAMA Netw Open. 2020. PMID: 32176304 Free PMC article.
-
A vicious circle between insulin resistance and inflammation in nonalcoholic fatty liver disease.Lipids Health Dis. 2017 Oct 16;16(1):203. doi: 10.1186/s12944-017-0572-9. Lipids Health Dis. 2017. PMID: 29037210 Free PMC article. Review.
-
Pro- and anti-inflammatory cytokines are the game-changers in childhood obesity-associated metabolic disorders (diabetes and non-alcoholic fatty liver diseases).Rev Endocr Metab Disord. 2024 Aug;25(4):783-803. doi: 10.1007/s11154-024-09884-y. Epub 2024 May 6. Rev Endocr Metab Disord. 2024. PMID: 38709387 Review.
Cited by
-
Association of Thallium with Diabetes Risk among Patients with Hearing Loss: Result from NHANES 2013 to 2018.Medicine (Baltimore). 2024 Mar 1;103(9):e37317. doi: 10.1097/MD.0000000000037317. Medicine (Baltimore). 2024. PMID: 38428895 Free PMC article.
-
Inflammation and the Potential Implication of Macrophage-Microglia Polarization in Human ASD: An Overview.Int J Mol Sci. 2023 Jan 31;24(3):2703. doi: 10.3390/ijms24032703. Int J Mol Sci. 2023. PMID: 36769026 Free PMC article. Review.
-
Prospective association between phthalate exposure in childhood and liver function in adolescence: the Ewha Birth and Growth Cohort Study.Environ Health. 2023 Jan 6;22(1):3. doi: 10.1186/s12940-022-00953-w. Environ Health. 2023. PMID: 36609289 Free PMC article.
-
Associations of metal mixtures with metabolic-associated fatty liver disease and non-alcoholic fatty liver disease: NHANES 2003-2018.Front Public Health. 2023 Mar 6;11:1133194. doi: 10.3389/fpubh.2023.1133194. eCollection 2023. Front Public Health. 2023. PMID: 36950101 Free PMC article.
-
GOS Ameliorates Nonalcoholic Fatty Liver Disease Induced by High Fat and High Sugar Diet through Lipid Metabolism and Intestinal Microbes.Nutrients. 2022 Jul 1;14(13):2749. doi: 10.3390/nu14132749. Nutrients. 2022. PMID: 35807929 Free PMC article.
References
-
- Mann JP, De Vito R, Mosca A, Alisi A, Armstrong MJ, Raponi M, Baumann U, et al.Portal inflammation is independently associated with fibrosis and metabolic syndrome in pediatric nonalcoholic fatty liver disease. HEPATOLOGY 2016;63:745–753. - PubMed
-
- Stefan N, Haring HU, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol 2018August30:pii: S2213–8587(2218)30154–30152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK106491/DK/NIDDK NIH HHS/United States
- R21 ES029681/ES/NIEHS NIH HHS/United States
- P01 ES022845/ES/NIEHS NIH HHS/United States
- R01 ES030691/ES/NIEHS NIH HHS/United States
- R21 ES028903/ES/NIEHS NIH HHS/United States
- R01 CA140561/CA/NCI NIH HHS/United States
- R01 DK117004/DK/NIDDK NIH HHS/United States
- R01 ES030364/ES/NIEHS NIH HHS/United States
- P30 DK048522/DK/NIDDK NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- P01 CA196569/CA/NCI NIH HHS/United States
- R01 ES029944/ES/NIEHS NIH HHS/United States
- P30 ES007048/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

