Rapid viscoelastic changes are a hallmark of early leukocyte activation
- PMID: 33730552
- PMCID: PMC8204340
- DOI: 10.1016/j.bpj.2021.02.042
Rapid viscoelastic changes are a hallmark of early leukocyte activation
Abstract
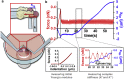
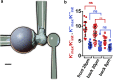
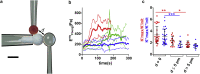
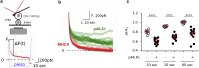
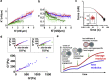
To accomplish their critical task of removing infected cells and fighting pathogens, leukocytes activate by forming specialized interfaces with other cells. The physics of this key immunological process are poorly understood, but it is important to understand them because leukocytes have been shown to react to their mechanical environment. Using an innovative micropipette rheometer, we show in three different types of leukocytes that, when stimulated by microbeads mimicking target cells, leukocytes become up to 10 times stiffer and more viscous. These mechanical changes start within seconds after contact and evolve rapidly over minutes. Remarkably, leukocyte elastic and viscous properties evolve in parallel, preserving a well-defined ratio that constitutes a mechanical signature specific to each cell type. Our results indicate that simultaneously tracking both elastic and viscous properties during an active cell process provides a new, to our knowledge, way to investigate cell mechanical processes. Our findings also suggest that dynamic immunomechanical measurements can help discriminate between leukocyte subtypes during activation.
Copyright © 2021 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Hochmuth R.M. Micropipette aspiration of living cells. J. Biomech. 2000;33:15–22. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

