The mitochondrial carrier SFXN1 is critical for complex III integrity and cellular metabolism
- PMID: 33730581
- PMCID: PMC8048093
- DOI: 10.1016/j.celrep.2021.108869
The mitochondrial carrier SFXN1 is critical for complex III integrity and cellular metabolism
Abstract
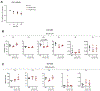
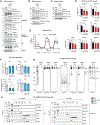
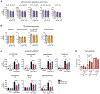
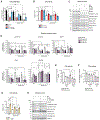
Mitochondrial carriers (MCs) mediate the passage of small molecules across the inner mitochondrial membrane (IMM), enabling regulated crosstalk between compartmentalized reactions. Despite MCs representing the largest family of solute carriers in mammals, most have not been subjected to a comprehensive investigation, limiting our understanding of their metabolic contributions. Here, we functionally characterize SFXN1, a member of the non-canonical, sideroflexin family. We find that SFXN1, an integral IMM protein with an uneven number of transmembrane domains, is a TIM22 complex substrate. SFXN1 deficiency leads to mitochondrial respiratory chain impairments, most detrimental to complex III (CIII) biogenesis, activity, and assembly, compromising coenzyme Q levels. The CIII dysfunction is independent of one-carbon metabolism, the known primary role for SFXN1 as a mitochondrial serine transporter. Instead, SFXN1 supports CIII function by participating in heme and α-ketoglutarate metabolism. Our findings highlight the multiple ways that SFXN1-based amino acid transport impacts mitochondrial and cellular metabolic efficiency.
Keywords: Complex III; OXPHOS; SFXN1; TIM22 complex; amino acid; heme; mitochondria; mitochondrial carrier; serine; sideroflexin.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

