Circuit reorganization in the Drosophila mushroom body calyx accompanies memory consolidation
- PMID: 33730583
- PMCID: PMC8515896
- DOI: 10.1016/j.celrep.2021.108871
Circuit reorganization in the Drosophila mushroom body calyx accompanies memory consolidation
Abstract
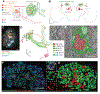
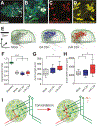
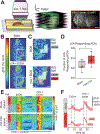
The formation and consolidation of memories are complex phenomena involving synaptic plasticity, microcircuit reorganization, and the formation of multiple representations within distinct circuits. To gain insight into the structural aspects of memory consolidation, we focus on the calyx of the Drosophila mushroom body. In this essential center, essential for olfactory learning, second- and third-order neurons connect through large synaptic microglomeruli, which we dissect at the electron microscopy level. Focusing on microglomeruli that respond to a specific odor, we reveal that appetitive long-term memory results in increased numbers of precisely those functional microglomeruli responding to the conditioned odor. Hindering memory consolidation by non-coincident presentation of odor and reward, by blocking protein synthesis, or by including memory mutants suppress these structural changes, revealing their tight correlation with the process of memory consolidation. Thus, olfactory long-term memory is associated with input-specific structural modifications in a high-order center of the fly brain.
Keywords: Drosophila; Kenyon cell; functional imaging; functional plasticity; memory consolidation; microglomerulus; mushroom body; mushroom body calyx; projection neuron; structural plasticity.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

