Organization and emergence of a mixed GABA-glycine retinal circuit that provides inhibition to mouse ON-sustained alpha retinal ganglion cells
- PMID: 33730586
- PMCID: PMC8030271
- DOI: 10.1016/j.celrep.2021.108858
Organization and emergence of a mixed GABA-glycine retinal circuit that provides inhibition to mouse ON-sustained alpha retinal ganglion cells
Abstract
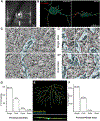
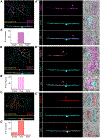
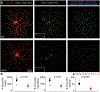
In the retina, amacrine interneurons inhibit retinal ganglion cell (RGC) dendrites to shape retinal output. Amacrine cells typically use either GABA or glycine to exert synaptic inhibition. Here, we combined transgenic tools with immunohistochemistry, electrophysiology, and 3D electron microscopy to determine the composition and organization of inhibitory synapses across the dendritic arbor of a well-characterized RGC type in the mouse retina: the ON-sustained alpha RGC. We find mixed GABA-glycine receptor synapses across this RGC type, unveiling the existence of "mixed" inhibitory synapses in the retinal circuit. Presynaptic amacrine boutons with dual release sites are apposed to ON-sustained alpha RGC postsynapses. We further reveal the sequence of postsynaptic assembly for these mixed synapses: GABA receptors precede glycine receptors, and a lack of early GABA receptor expression impedes the recruitment of glycine receptors. Together our findings uncover the organization and developmental profile of an additional motif of inhibition in the mammalian retina.
Keywords: GABA receptors; amacrine cells; glycine receptors; inhibition; retinal ganglion cell; synapses.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
-
- Bishop D, Nikić I, Brinkoetter M, Knecht S, Potz S, Kerschensteiner M, and Misgeld T (2011). Near-infrared branding efficiently correlates light and electron microscopy. Nat. Methods 8, 568–570. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

