Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera
- PMID: 33730597
- PMCID: PMC7901269
- DOI: 10.1016/j.cell.2021.02.037
Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera
Abstract
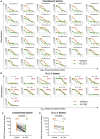
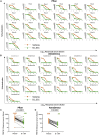
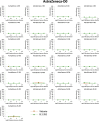
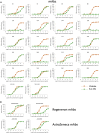
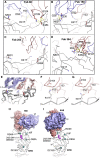
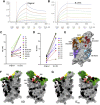
The race to produce vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) began when the first sequence was published, and this forms the basis for vaccines currently deployed globally. Independent lineages of SARS-CoV-2 have recently been reported: UK, B.1.1.7; South Africa, B.1.351; and Brazil, P.1. These variants have multiple changes in the immunodominant spike protein that facilitates viral cell entry via the angiotensin-converting enzyme-2 (ACE2) receptor. Mutations in the receptor recognition site on the spike are of great concern for their potential for immune escape. Here, we describe a structure-function analysis of B.1.351 using a large cohort of convalescent and vaccinee serum samples. The receptor-binding domain mutations provide tighter ACE2 binding and widespread escape from monoclonal antibody neutralization largely driven by E484K, although K417N and N501Y act together against some important antibody classes. In a number of cases, it would appear that convalescent and some vaccine serum offers limited protection against this variant.
Keywords: ACE2; B.1.351; SARS-CoV-2; South Africa; antibody; escape; neutralization; receptor-binding domain; vaccine; variant.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests G.R.S. sits on the GSK Vaccines Scientific Advisory Board. Oxford University holds intellectual property related to the Oxford-AstraZeneca vaccine. A.J.P. is Chair of UK Department Health and Social Care’s (DHSC) Joint Committee on Vaccination & Immunisation (JCVI), but does not participate in the JCVI COVID-19 committee, and is a member of the WHO’s SAGE. The views expressed in this article do not necessarily represent the views of DHSC, JCVI, or WHO. The University of Oxford has entered into a partnership with AstraZeneca on coronavirus vaccine development. The University of Oxford has protected intellectual property disclosed in this publication.
Figures


References
-
- Aricescu A.R., Lu W., Jones E.Y. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr D Biol Crystallogr. 2006;62:1243–1250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

