Impact of COVID-19-related care disruptions on cervical cancer screening in the United States
- PMID: 33730899
- PMCID: PMC8484743
- DOI: 10.1177/09691413211001097
Impact of COVID-19-related care disruptions on cervical cancer screening in the United States
Abstract
Objectives: To quantify the secondary impacts of the COVID-19 pandemic disruptions to cervical cancer screening in the United States, stratified by step in the screening process and primary test modality, on cervical cancer burden.
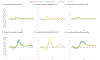
Methods: We conducted a comparative model-based analysis using three independent NCI Cancer Intervention and Surveillance Modeling Network cervical models to quantify the impact of eight alternative COVID-19-related screening disruption scenarios compared to a scenario of no disruptions. Scenarios varied by the duration of the disruption (6 or 24 months), steps in the screening process being disrupted (primary screening, surveillance, colposcopy, excisional treatment), and primary screening modality (cytology alone or cytology plus human papillomavirus "cotesting").
Results: The models consistently showed that COVID-19-related disruptions yield small net increases in cervical cancer cases by 2027, which are greater for women previously screened with cytology compared with cotesting. When disruptions affected all four steps in the screening process under cytology-based screening, there were an additional 5-7 and 38-45 cases per one million screened for 6- and 24-month disruptions, respectively. In contrast, under cotesting, there were additional 4-5 and 35-45 cases per one million screened for 6- and 24-month disruptions, respectively. The majority (58-79%) of the projected increases in cases under cotesting were due to disruptions to surveillance, colposcopies, or excisional treatment, rather than to primary screening.
Conclusions: Women in need of surveillance, colposcopies, or excisional treatment, or whose last primary screen did not involve human papillomavirus testing, may comprise priority groups for reintroductions.
Keywords: COVID-19; cervical cancer screening; simulation modeling.
Conflict of interest statement
Disclosures
All other authors declare no conflicts.
Figures
References
-
- American Cancer Socity. “Key Statistics for Cervical Cancer” Available at: https://www.cancer.org/cancer/cervical-cancer/about/key-statistics.html (accessed August 19, 2020).
-
- Mast C, Munoz del Rio A. Delayed cancer screenings—a second look. Verona, WI: Epic Health Research Network; 2020. https://ehrn.org/articles/delayed-cancer-screenings-a-second-lookexternalAccessed February 5, 2021.
-
- United Nations Population Division. World Population Prospects: The 2017 Revision.[Online] Accessed 18 December 2017. Available at: https://esa.un.org/unpd/wpp/.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

