Maternal and neonatal data collection systems in low- and middle-income countries for maternal vaccines active safety surveillance systems: A scoping review
- PMID: 33731029
- PMCID: PMC7968860
- DOI: 10.1186/s12884-021-03686-9
Maternal and neonatal data collection systems in low- and middle-income countries for maternal vaccines active safety surveillance systems: A scoping review
Abstract
Background: Most post-licensure vaccine pharmacovigilance in low- and middle-income countries (LMICs) are passive reporting systems. These have limited utility for maternal immunization pharmacovigilance in LMIC settings and need to be supplemented with active surveillance. Our study's main objective was to identify existing perinatal data collection systems in LMICs that collect individual information on maternal and neonatal health outcomes and could be developed to inform active safety surveillance of novel vaccines for use during pregnancy.
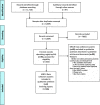
Methods: A scoping review was performed following the Arksey and O'Malley six-stage approach. We included studies describing electronic or mixed paper-electronic data collection systems in LMICs, including research networks, electronic medical records, and custom software platforms for health information systems. Medline PubMed, EMBASE, Global Health, Cochrane Library, LILACS, Bibliography of Asian Studies (BAS), and CINAHL were searched through August 2019. We also searched grey literature including through Google and websites of existing relevant perinatal data collection systems, as well as contacted authors of key studies and experts in the field to validate the information and identify additional sources of relevant unpublished information.
Results: A total of 11,817 records were identified. The full texts of 264 records describing 96 data collection systems were assessed for eligibility. Eight perinatal data collection systems met our inclusion criteria: Global Network's Maternal Newborn Health Registry, International Network for the Demographic Evaluation of Populations and their Health; Perinatal Informatic System; Pregnancy Exposure Registry & Birth Defects Surveillance; SmartCare; Open Medical Record System; Open Smart Register Platform and District Health Information Software 2. These selected systems were qualitatively characterized according to seven different domains: governance; system design; system management; data management; data sources, outcomes and data quality.
Conclusion: This review provides a list of active maternal and neonatal data collection systems in LMICs and their characteristics as well as their outreach, strengths, and limitations. Findings could potentially help further understand where to obtain population-based high-quality information on outcomes to inform the conduct of maternal immunization active vaccine safety surveillance activities and research in LMICs.
Keywords: Active surveillance; Electronic Registries; Global Health; Health Information Systems; Pharmacovigilance; Pregnancy; Vaccination.
Conflict of interest statement
The authors take sole responsibility for the writing and content of the paper. All authors have nothing to disclose.
Figures
References
-
- GAPPS. Maternal Immunization Safety Monitoring In Low- and- Middle-income Countries: A Roadmap For Program Development. Global Alliance to Prevent Prematurity and Stillbirth. 2017. https://www.gapps.org/PDF/MaternalImmunizationSafetyMonitoringInLMICs.pdf. Accessed 19 May 2020.
-
- Lackritz E, Stepanchak M: Maternal Immunization Safety Monitoring in Low- and Middle-Income Countries: A Roadmap for Program Development. 2017. https://www.gapps.org/PDF/MaternalImmunizationSafetyMonitoringInLMICs.pdf. Accessed 3 July 2020.
-
- Jones CE, Munoz FM, Spiegel HM, Heininger U, Zuber PL, Edwards KM, Lambach P, Neels P, Kohl KS, Gidudu J, et al. Guideline for collection, analysis and presentation of safety data in clinical trials of vaccines in pregnant women. Vaccine. 2016;34(49):5998–6006. doi: 10.1016/j.vaccine.2016.07.032. - DOI - PMC - PubMed
-
- Kochhar S, Bonhoeffer J, Jones CE, Muñoz FM, Honrado A, Bauwens J, Sobanjo-Ter Meulen A, Hirschfeld S. Immunization in pregnancy clinical research in low- and middle-income countries - Study design, regulatory and safety considerations. Vaccine. 2017;35(48 Pt A):6575–6581. doi: 10.1016/j.vaccine.2017.03.103. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous