Neoadjuvant chemoradiotherapy improves survival in locally advanced adenocarcinoma of esophagogastric junction compared with neoadjuvant chemotherapy: a propensity score matching analysis
- PMID: 33731072
- PMCID: PMC7972233
- DOI: 10.1186/s12893-021-01136-z
Neoadjuvant chemoradiotherapy improves survival in locally advanced adenocarcinoma of esophagogastric junction compared with neoadjuvant chemotherapy: a propensity score matching analysis
Abstract
Background: To analyze whether neoadjuvant chemoradiotherapy (nCRT) could improve the survival for patients with adenocarcinoma of the esophagogastric junction compared with neoadjuvant chemotherapy (nCT). Both neoadjuvant chemotherapy alone and chemoradiotherapy before surgery have been shown to improve overall long-term survival for patients with adenocarcinoma in the esophagus or esophagogastric junction compared to surgery alone. It remains controversial whether nCRT is superior to nCT.
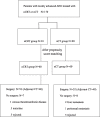
Methods: 170 Patients with locally advanced (cT3-4NxM0) Siewert II and III adenocarcinoma of the esophagogastric junction (AEG) were treated with neoadjuvant chemotherapy consisting of capecitabine plus oxaliplatin with or without concurrent radiotherapy in the Fourth Hospital of Hebei Medical University. Intensity-modulated radiation therapy (IMRT) was used and delivered in 5 daily fractions of 1.8 Gy per week for 5 weeks (total dose of PTV: 45 Gy). 120 Patients were included in the propensity score matching (PSM) analysis to compare the effects of nCRT with nCT on survival.
Results: With a median follow-up of 41.2 months for patients alive after propensity score matching analysis, the 1- and 3-year OS were 84.8%, 55.0% in nCRT group and 78.3%, 38.3% in nCT group (P = 0.040; HR = 1.65, 95% CI 1.02-2.69). The 1- and 3-year PFS were 84.9%, 49.2% in nCRT group and 68.3%, 29.0% in nCT group (P = 0.010; HR = 1.80, 95% CI 1.14-2.85). The pathological complete response (pCR) was 17.0% in nCRT group and 1.9% in nCT group (P = 0.030). No significant difference was observed in postoperative complications between the two groups.
Conclusion: The nCRT confers a better survival with improved R0 resection rate and pCR rate compared with nCT for the patients with locally advanced AEG.
Keywords: Adenocarcinoma of the esophagogastric junction; Chemoradiotherapy; Chemotherapy; Neoadjuvant treatment; Propensity score matching analysis.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures
References
-
- Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28(35):5210–5218. doi: 10.1200/JCO.2009.26.6114. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous