Estimating the potential impact of Attractive Targeted Sugar Baits (ATSBs) as a new vector control tool for Plasmodium falciparum malaria
- PMID: 33731111
- PMCID: PMC7968277
- DOI: 10.1186/s12936-021-03684-4
Estimating the potential impact of Attractive Targeted Sugar Baits (ATSBs) as a new vector control tool for Plasmodium falciparum malaria
Abstract
Background: Attractive targeted sugar baits (ATSBs) are a promising new tool for malaria control as they can target outdoor-feeding mosquito populations, in contrast to current vector control tools which predominantly target indoor-feeding mosquitoes.
Methods: It was sought to estimate the potential impact of these new tools on Plasmodium falciparum malaria prevalence in African settings by combining data from a recent entomological field trial of ATSBs undertaken in Mali with mathematical models of malaria transmission. The key parameter determining impact on the mosquito population is the excess mortality due to ATSBs, which is estimated from the observed reduction in mosquito catch numbers. A mathematical model capturing the life cycle of P. falciparum malaria in mosquitoes and humans and incorporating the excess mortality was used to estimate the potential epidemiological effect of ATSBs.
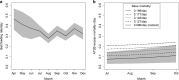
Results: The entomological study showed a significant reduction of ~ 57% (95% CI 33-72%) in mosquito catch numbers, and a larger reduction of ~ 89% (95% CI 75-100%) in the entomological inoculation rate due to the fact that, in the presence of ATSBs, most mosquitoes do not live long enough to transmit malaria. The excess mortality due to ATSBs was estimated to be lower (mean 0.09 per mosquito per day, seasonal range 0.07-0.11 per day) than the bait feeding rate obtained from one-day staining tests (mean 0.34 per mosquito per day, seasonal range 0.28-0.38 per day).
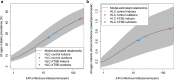
Conclusions: From epidemiological modelling, it was predicted that ATSBs could result in large reductions (> 30% annually) in prevalence and clinical incidence of malaria, even in regions with an existing high malaria burden. These results suggest that this new tool could provide a promising addition to existing vector control tools and result in significant reductions in malaria burden across a range of malaria-endemic settings.
Keywords: Malaria; Mosquito; Vector control.
Conflict of interest statement
We declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

