Fibronectin as a multiregulatory molecule crucial in tumor matrisome: from structural and functional features to clinical practice in oncology
- PMID: 33731188
- PMCID: PMC7972229
- DOI: 10.1186/s13046-021-01908-8
Fibronectin as a multiregulatory molecule crucial in tumor matrisome: from structural and functional features to clinical practice in oncology
Abstract
Deciphering extracellular matrix (ECM) composition and architecture may represent a novel approach to identify diagnostic and therapeutic targets in cancer. Among the ECM components, fibronectin and its fibrillary assembly represent the scaffold to build up the entire ECM structure, deeply affecting its features. Herein we focus on this extraordinary protein starting from its complex structure and defining its role in cancer as prognostic and theranostic marker.
Keywords: Actin cytoskeleton; Cancer; Cancer-associated fibroblasts; Dormant cells; Extracellular matrix; Extracellular vesicles; Fibrillogenesis; Fibronectin; Metastasis; Metastatic niche.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

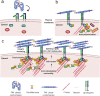
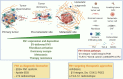
References
-
- Pearce OMT, Delaine-Smith RM, Maniati E, Nichols S, Wang J, Böhm S, et al. Deconstruction of a metastatic tumor microenvironment reveals a common matrix response in human cancers. Cancer Discov. 2018;8(3):304–319. doi: 10.1158/2159-8290.CD-17-0284. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

