Development of the Canadian COVID-19 Emergency Department Rapid Response Network population-based registry: a methodology study
- PMID: 33731427
- PMCID: PMC8096396
- DOI: 10.9778/cmajo.20200290
Development of the Canadian COVID-19 Emergency Department Rapid Response Network population-based registry: a methodology study
Abstract
Background: Emergency physicians lack high-quality evidence for many diagnostic and treatment decisions made for patients with suspected or confirmed coronavirus disease 2019 (COVID-19). Our objective is to describe the methods used to collect and ensure the data quality of a multicentre registry of patients presenting to the emergency department with suspected or confirmed COVID-19.
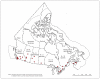
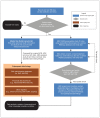
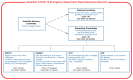
Methods: This methodology study describes a population-based registry that has been enrolling consecutive patients presenting to the emergency department with suspected or confirmed COVID-19 since Mar. 1, 2020. Most data are collected from retrospective chart review. Phone follow-up with patients at 30 days captures the World Health Organization clinical improvement scale and contextual, social and cultural variables. Phone follow-up also captures patient-reported quality of life using the Veterans Rand 12-Item Health Survey at 30 days, 60 days, 6 months and 12 months. Fifty participating emergency departments from 8 provinces in Canada currently enrol patients into the registry.
Interpretation: Data from the registry of the Canadian COVID-19 Emergency Department Rapid Response Network will be used to derive and validate clinical decision rules to inform clinical decision-making, describe the natural history of the disease, evaluate COVID-19 diagnostic tests and establish the real-world effectiveness of treatments and vaccines, including in populations that are excluded or underrepresented in clinical trials. This registry has the potential to generate scientific evidence to inform our pandemic response, and to serve as a model for the rapid implementation of population-based data collection protocols for future public health emergencies.
Trial registration: Clinicaltrials.gov, no. NCT04702945.
© 2021 Joule Inc. or its licensors.
Conflict of interest statement
Competing interests: Patrick Fok is a shareholder of Hologic, Merck Pharmaceuticals and Moderna. Brian Rowe is the Scientific Director of the Institute of Circulatory and Respiratory Health at the Canadian Institutes of Health Research (CIHR) and reports grants and salary from the CIHR outside the submitted work. Hana Wiemer reports grants and nonfinancial support from Purdue Pharma Canada outside the submitted work. Justin Yan reports grants from Government of Ontario Ministry of Colleges and Universities, during the conduct of the study. No other competing interests were declared.
Figures
References
-
- WHO coronavirus disease (COVID-19) dashboard. Geneva: World Health Organization; [accessed 2021 Mar. 2]. Available https://covid19.who.int/?gclid=CjwKCAjw5Kv7BRBSEiwAXGDEle_dyJCA3TPMvUjiK....
-
- COVID-19 map FAQs. Baltimore (MD): Johns Hopkins University Center for Systems Science and Engineering; [accessed 2020 Mar. 30]. updated 2020 Dec 1. Available https://systems.jhu.edu/research/public-health/2019-ncov-map-faqs/
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
