Reduced Repetition Suppression in Aging is Driven by Tau-Related Hyperactivity in Medial Temporal Lobe
- PMID: 33731446
- PMCID: PMC8084317
- DOI: 10.1523/JNEUROSCI.2504-20.2021
Reduced Repetition Suppression in Aging is Driven by Tau-Related Hyperactivity in Medial Temporal Lobe
Abstract
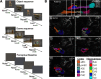
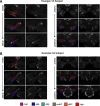
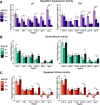
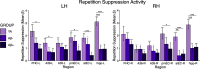
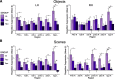
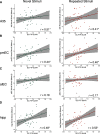
Tau deposition begins in the medial temporal lobe (MTL) in aging and Alzheimer's disease (AD), and MTL neural dysfunction is commonly observed in these groups. However, the association between tau and MTL neural activity has not been fully characterized. We investigated the effects of tau on repetition suppression, the reduction of activity for repeated stimulus presentations compared to novel stimuli. We used task-based functional MRI (fMRI) to assess MTL subregional activity in 21 young adults (YA) and 45 cognitively normal human older adults (OA; total sample: 37 females, 29 males). AD pathology was measured with position emission tomography (PET), using 18F-Flortaucipir for tau and 11C-Pittsburgh compound B (PiB) for amyloid-β (Aβ). The MTL was segmented into six subregions using high-resolution structural images. We compared the effects of low tau pathology, restricted to entorhinal cortex and hippocampus (Tau- OA), to high tau pathology, also occurring in temporal and limbic regions (Tau+ OA). Low levels of tau (Tau- OA vs YA) were associated with reduced repetition suppression activity specifically in anterolateral entorhinal cortex (alEC) and hippocampus, the first regions to accumulate tau. High tau pathology (Tau+ vs Tau- OA) was associated with widespread reductions in repetition suppression across MTL. Further analyses indicated that reduced repetition suppression was driven by hyperactivity to repeated stimuli, rather than decreased activity to novel stimuli. Increased activation was associated with entorhinal tau, but not Aβ. These findings reveal a link between tau deposition and neural dysfunction in MTL, in which tau-related hyperactivity prevents deactivation to repeated stimuli, leading to reduced repetition suppression.SIGNIFICANCE STATEMENT Abnormal neural activity occurs in the medial temporal lobe (MTL) in aging and Alzheimer's disease (AD). Because tau pathology first deposits in the MTL in aging, this altered activity may be due to local tau pathology, and distinct MTL subregions may be differentially vulnerable. We demonstrate that in older adults (OAs) with low tau pathology, there are focal alterations in activity in MTL subregions that first develop tau pathology, while OAs with high tau pathology have aberrant activity throughout MTL. Tau was associated with hyperactivity to repeated stimulus presentations, leading to reduced repetition suppression, the discrimination between novel and repeated stimuli. Our data suggest that tau deposition is related to abnormal activity in MTL before the onset of cognitive decline.
Keywords: Alzheimer's disease; PET; aging; fMRI; repetition suppression; tau.
Copyright © 2021 the authors.
Figures
References
-
- Angulo SL, Orman R, Neymotin SA, Liu L, Buitrago L, Cepeda-Prado E, Stefanov D, Lytton WW, Stewart M, Small SA, Duff KE, Moreno H (2017) Tau and amyloid-related pathologies in the entorhinal cortex have divergent effects in the hippocampal circuit. Neurobiol Dis 108:261–276. 10.1016/j.nbd.2017.08.015 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
