Reduced differentiation of intestinal epithelial cells in wasting marmoset syndrome
- PMID: 33731497
- PMCID: PMC8182325
- DOI: 10.1292/jvms.20-0532
Reduced differentiation of intestinal epithelial cells in wasting marmoset syndrome
Abstract
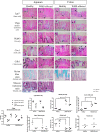
Wasting marmoset syndrome (WMS) is a serious disease in captive common marmoset (Callithrix jacchus) colonies. Because of the high mortality rates, elucidation of the underlying mechanisms is essential. In this study, we compared the histopathology, the number of each epithelial cell in the jejunum and colon, and the expression patterns of some molecular markers between healthy and WMS-affected marmosets. Atrophy of villi in the jejunum and mononuclear cell infiltration in the lamina propria were observed in the intestinal tract of WMS-affected marmosets. Although the numbers of transient amplifying cells and tuft cells were increased, the number of goblet cells was obviously decreased in the jejunum and colon of WMS-affected marmosets compared to healthy marmosets. In addition, the number of enterocytes in the jejunum was decreased in WMS animals. There was no apparent difference in the numbers of stem cells, enteroendocrine cells, or Paneth cells. The expression of β-catenin and Tcf7l2 was increased in WMS, and the co-existence of β-catenin and Tcf7l2/Cyclin D1 was observed around the crypts in WMS-affected marmosets. These findings suggest that cell proliferation continues, but cell differentiation is halted in the intestinal tract due to the enhanced β-catenin/Tcf7l2/Cyclin D1signaling pathway in WMS, which results in malfunction of the villus and mucosa.
Keywords: common marmoset; differentiation; intestinal epithelial cell; wasting marmoset syndrome.
Conflict of interest statement
The authors have nothing to disclose.
Figures


References
-
- Abbott D. H., Barnett D. K., Colman R. J., Yamamoto M. E., Schultz-Darken N. J.2003. Aspects of common marmoset basic biology and life history important for biomedical research. Comp. Med. 53: 339–350. - PubMed
-
- Batlle E., Henderson J. T., Beghtel H., van den Born M. M. W., Sancho E., Huls G., Meeldijk J., Robertson J., van de Wetering M., Pawson T., Clevers H.2002. β-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell 111: 251–263. doi: 10.1016/S0092-8674(02)01015-2 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

