Chondrogenesis mediates progression of ankylosing spondylitis through heterotopic ossification
- PMID: 33731675
- PMCID: PMC7969928
- DOI: 10.1038/s41413-021-00140-6
Chondrogenesis mediates progression of ankylosing spondylitis through heterotopic ossification
Abstract
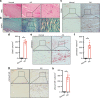
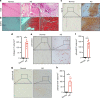
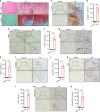
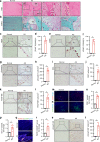
Ankylosing spondylitis (AS) is chronic inflammatory arthritis with a progressive fusion of axial joints. Anti-inflammatory treatments such as anti-TNF-α antibody therapy suppress inflammation but do not effectively halt the progression of spine fusion in AS patients. Here we report that the autoimmune inflammation of AS generates a microenvironment that promotes chondrogenesis in spine ligaments as the process of spine fusion. Chondrocyte differentiation was observed in the ligaments of patients with early-stage AS, and cartilage formation was followed by calcification. Moreover, a large number of giant osteoclasts were found in the inflammatory environment of ligaments and on bony surfaces of calcified cartilage. Resorption activity by these giant osteoclasts generated marrow with high levels of active TGF-β, which induced new bone formation in the ligaments. Notably, no Osterix+ osteoprogenitors were found in osteoclast resorption areas, indicating uncoupled bone resorption and formation. Even at the late and maturation stages, the uncoupled osteoclast resorption in bony interspinous ligament activates TGF-β to induce the progression of ossification in AS patients. Osteoclast resorption of calcified cartilage-initiated ossification in the progression of AS is a similar pathologic process of acquired heterotopic ossification (HO). Our finding of cartilage formation in the ligaments of AS patients revealed that the pathogenesis of spinal fusion is a process of HO and explained why anti-inflammatory treatments do not slow ankylosing once there is new bone formation in spinal soft tissues. Thus, inhibition of HO formation, such as osteoclast activity, cartilage formation, or TGF-β activity could be a potential therapy for AS.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Sieper J, et al. Persistent reduction of spinal inflammation as assessed by magnetic resonance imaging in patients with ankylosing spondylitis after 2 years of treatment with the anti-tumour necrosis factor agent infliximab. Rheumatology (Oxford) 2005;44:1525–1530. doi: 10.1093/rheumatology/kei046. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

