Subchondral bone microenvironment in osteoarthritis and pain
- PMID: 33731688
- PMCID: PMC7969608
- DOI: 10.1038/s41413-021-00147-z
Subchondral bone microenvironment in osteoarthritis and pain
Abstract
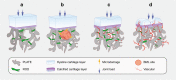
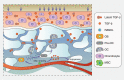
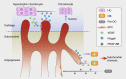
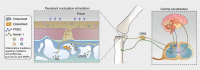
Osteoarthritis comprises several joint disorders characterized by articular cartilage degeneration and persistent pain, causing disability and economic burden. The incidence of osteoarthritis is rapidly increasing worldwide due to aging and obesity trends. Basic and clinical research on osteoarthritis has been carried out for decades, but many questions remain unanswered. The exact role of subchondral bone during the initiation and progression osteoarthritis remains unclear. Accumulating evidence shows that subchondral bone lesions, including bone marrow edema and angiogenesis, develop earlier than cartilage degeneration. Clinical interventions targeting subchondral bone have shown therapeutic potential, while others targeting cartilage have yielded disappointing results. Abnormal subchondral bone remodeling, angiogenesis and sensory nerve innervation contribute directly or indirectly to cartilage destruction and pain. This review is about bone-cartilage crosstalk, the subchondral microenvironment and the critical role of both in osteoarthritis progression. It also provides an update on the pathogenesis of and interventions for osteoarthritis and future research targeting subchondral bone.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
Grants and funding
- 2018YFC2001500/Ministry of Science and Technology of the People's Republic of China (Chinese Ministry of Science and Technology)
- 91749204;81771491;81871099/National Natural Science Foundation of China (National Science Foundation of China)
- 81871099/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

