D-Aspartate consumption selectively promotes intermediate-term spatial memory and the expression of hippocampal NMDA receptor subunits
- PMID: 33731750
- PMCID: PMC7969773
- DOI: 10.1038/s41598-021-85360-w
D-Aspartate consumption selectively promotes intermediate-term spatial memory and the expression of hippocampal NMDA receptor subunits
Abstract
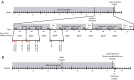
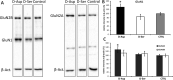
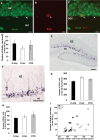
D-Aspartate (D-Asp) and D-serine (D-Ser) have been proposed to promote early-phase LTP in vitro and to enhance spatial memory in vivo. Here, we investigated the behavioural effects of chronic consumption of D-Asp and D-Ser on spatial learning of mice together with the expression of NMDA receptors. We also studied the alterations of neurogenesis by morphometric analysis of bromo-deoxyuridine incorporating and doublecortin expressing cells in the hippocampus. Our results specify a time period (3-4 h post-training), within which the animals exposed to D-Asp (but not D-Ser) show a more stable memory during retrieval. The cognitive improvement is due to elimination of transient bouts of destabilization and reconsolidation of memory, rather than to enhanced acquisition. D-Asp also protracted reversal learning probably due to reduced plasticity. Expression of GluN1 and GluN2A subunits was elevated in the hippocampus of D-Asp (but not D-Ser) treated mice. D-Asp or D-Ser did not alter the proliferation of neuronal progenitor cells in the hippocampus. The observed learning-related changes evoked by D-Asp are unlikely to be due to enhanced proliferation and recruitment of new neurones. Rather, they are likely associated with an upregulation of NMDA receptors, as well as a reorganization of receptor subunit assemblies in existing hippocampal/dentate neurons.
Conflict of interest statement
The authors declare no competing interests.
Figures

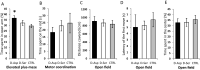
Similar articles
-
Ras inhibitor S-trans, trans-farnesylthiosalicylic acid enhances spatial memory and hippocampal long-term potentiation via up-regulation of NMDA receptor.Neuropharmacology. 2018 Sep 1;139:257-267. doi: 10.1016/j.neuropharm.2018.03.026. Epub 2018 Mar 22. Neuropharmacology. 2018. PMID: 29578035
-
Long-term saturated fat-enriched diets impair hippocampal learning and memory processes in a sex-dependent manner.Neuropharmacology. 2024 Nov 15;259:110108. doi: 10.1016/j.neuropharm.2024.110108. Epub 2024 Aug 10. Neuropharmacology. 2024. PMID: 39128582
-
GluN2B-containing NMDA receptors contribute to the beneficial effects of hydrogen sulfide on cognitive and synaptic plasticity deficits in APP/PS1 transgenic mice.Neuroscience. 2016 Oct 29;335:170-83. doi: 10.1016/j.neuroscience.2016.08.033. Epub 2016 Aug 28. Neuroscience. 2016. PMID: 27581687
-
Plasticity, hippocampal place cells, and cognitive maps.Arch Neurol. 2001 Jun;58(6):874-81. doi: 10.1001/archneur.58.6.874. Arch Neurol. 2001. PMID: 11405801 Review.
-
New insights on the role of free D-aspartate in the mammalian brain.Amino Acids. 2012 Nov;43(5):1861-71. doi: 10.1007/s00726-012-1356-1. Epub 2012 Aug 1. Amino Acids. 2012. PMID: 22851050 Review.
Cited by
-
Milk phospholipid-coated lipid droplets modulate the infant gut microbiota and metabolome influencing weight gain.Microbiome. 2025 May 14;13(1):120. doi: 10.1186/s40168-025-02106-w. Microbiome. 2025. PMID: 40369689 Free PMC article. Clinical Trial.
-
Predicting performance in attention by measuring key metabolites in the PCC with 7T MRS.Sci Rep. 2024 Jul 24;14(1):17099. doi: 10.1038/s41598-024-67866-1. Sci Rep. 2024. PMID: 39048626 Free PMC article.
-
Evolution and function of neurocognitive systems in non-human animals.Sci Rep. 2021 Dec 8;11(1):23487. doi: 10.1038/s41598-021-02736-8. Sci Rep. 2021. PMID: 34880266 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

