Adaptive constrained constructive optimisation for complex vascularisation processes
- PMID: 33731776
- PMCID: PMC7969782
- DOI: 10.1038/s41598-021-85434-9
Adaptive constrained constructive optimisation for complex vascularisation processes
Abstract
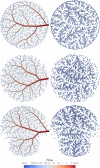
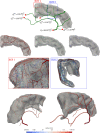
Mimicking angiogenetic processes in vascular territories acquires importance in the analysis of the multi-scale circulatory cascade and the coupling between blood flow and cell function. The present work extends, in several aspects, the Constrained Constructive Optimisation (CCO) algorithm to tackle complex automatic vascularisation tasks. The main extensions are based on the integration of adaptive optimisation criteria and multi-staged space-filling strategies which enhance the modelling capabilities of CCO for specific vascular architectures. Moreover, this vascular outgrowth can be performed either from scratch or from an existing network of vessels. Hence, the vascular territory is defined as a partition of vascular, avascular and carriage domains (the last one contains vessels but not terminals) allowing one to model complex vascular domains. In turn, the multi-staged space-filling approach allows one to delineate a sequence of biologically-inspired stages during the vascularisation process by exploiting different constraints, optimisation strategies and domain partitions stage by stage, improving the consistency with the architectural hierarchy observed in anatomical structures. With these features, the aDaptive CCO (DCCO) algorithm proposed here aims at improving the modelled network anatomy. The capabilities of the DCCO algorithm are assessed with a number of anatomically realistic scenarios.
Conflict of interest statement
The authors declare no competing interests.
Figures








References
-
- Jain R. Determinants of tumor blood flow: a review. Cancer Res. 1988;48:2641–2658. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

