Interleukin-10 suppression enhances T-cell antitumor immunity and responses to checkpoint blockade in chronic lymphocytic leukemia
- PMID: 33731852
- PMCID: PMC8446094
- DOI: 10.1038/s41375-021-01217-1
Interleukin-10 suppression enhances T-cell antitumor immunity and responses to checkpoint blockade in chronic lymphocytic leukemia
Abstract
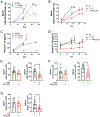
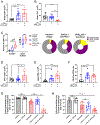
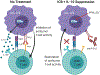
T-cell dysfunction is a hallmark of B-cell Chronic Lymphocytic Leukemia (CLL), where CLL cells downregulate T-cell responses through regulatory molecules including programmed death ligand-1 (PD-L1) and Interleukin-10 (IL-10). Immune checkpoint blockade (ICB) aims to restore T-cell function by preventing the ligation of inhibitory receptors like PD-1. However, most CLL patients do not respond well to this therapy. Thus, we investigated whether IL-10 suppression could enhance antitumor T-cell activity and responses to ICB. Since CLL IL-10 expression depends on Sp1, we utilized a novel, better tolerated analogue of the Sp1 inhibitor mithramycin (MTMox32E) to suppress CLL IL-10. MTMox32E treatment inhibited mouse and human CLL IL-10 production and maintained T-cell effector function in vitro. In the Eμ-Tcl1 mouse model, treatment reduced plasma IL-10 and CLL burden and increased CD8+ T-cell proliferation, effector and memory cell prevalence, and interferon-γ production. When combined with ICB, suppression of IL-10 improved responses to anti-PD-L1 as shown by a 4.5-fold decrease in CLL cell burden compared to anti-PD-L1 alone. Combination therapy also produced more interferon-γ+, cytotoxic effector KLRG1+, and memory CD8+ T-cells, and fewer exhausted T-cells. Since current therapies for CLL do not target IL-10, this provides a novel strategy to improve immunotherapies.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing Interests:
J.S.T. is a co-founder of Centrose (Madision, WI, USA). No other authors have competing financial interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

