Lysosomal cholesterol accumulation contributes to the movement phenotypes associated with NUS1 haploinsufficiency
- PMID: 33731878
- PMCID: PMC8263489
- DOI: 10.1038/s41436-021-01137-6
Lysosomal cholesterol accumulation contributes to the movement phenotypes associated with NUS1 haploinsufficiency
Abstract
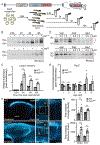
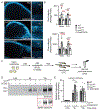
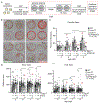
Purpose: Variants in NUS1 are associated with a congenital disorder of glycosylation, developmental and epileptic encephalopathies, and are possible contributors to Parkinson disease pathogenesis. How the diverse functions of the NUS1-encoded Nogo B receptor (NgBR) relate to these different phenotypes is largely unknown. We present three patients with de novo heterozygous variants in NUS1 that cause a complex movement disorder, define pathogenic mechanisms in cells and zebrafish, and identify possible therapy.
Methods: Comprehensive functional studies were performed using patient fibroblasts, and a zebrafish model mimicking NUS1 haploinsufficiency.
Results: We show that de novo NUS1 variants reduce NgBR and Niemann-Pick type C2 (NPC2) protein amount, impair dolichol biosynthesis, and cause lysosomal cholesterol accumulation. Reducing nus1 expression 50% in zebrafish embryos causes abnormal swim behaviors, cholesterol accumulation in the nervous system, and impaired turnover of lysosomal membrane proteins. Reduction of cholesterol buildup with 2-hydroxypropyl-ß-cyclodextrin significantly alleviates lysosomal proteolysis and motility defects.
Conclusion: Our results demonstrate that these NUS1 variants cause multiple lysosomal phenotypes in cells. We show that the movement deficits associated with nus1 reduction in zebrafish arise in part from defective efflux of cholesterol from lysosomes, suggesting that treatments targeting cholesterol accumulation could be therapeutic.
Conflict of interest statement
Conflict of Interest Statement
All the authors declare that they have no competing or financial conflicts of interest.
Declaration of interests
The authors declare no competing interests.
Figures


Similar articles
-
Niemann-Pick C-like Endolysosomal Dysfunction in DHDDS Patient Cells, a Congenital Disorder of Glycosylation, Can Be Treated with Miglustat.Int J Mol Sci. 2025 Feb 10;26(4):1471. doi: 10.3390/ijms26041471. Int J Mol Sci. 2025. PMID: 40003936 Free PMC article.
-
The role of Niemann-Pick type C2 in zebrafish embryonic development.Development. 2021 Apr 1;148(7):dev194258. doi: 10.1242/dev.194258. Epub 2021 Apr 15. Development. 2021. PMID: 33722902 Free PMC article.
-
Loss of Drosophila NUS1 results in cholesterol accumulation and Parkinson's disease-related neurodegeneration.FASEB J. 2022 Jul;36(7):e22411. doi: 10.1096/fj.202200212R. FASEB J. 2022. PMID: 35695805
-
Research advances on neurite outgrowth inhibitor B receptor.J Cell Mol Med. 2020 Jul;24(14):7697-7705. doi: 10.1111/jcmm.15391. Epub 2020 Jun 15. J Cell Mol Med. 2020. PMID: 32542927 Free PMC article. Review.
-
DHDDS and NUS1: A Converging Pathway and Common Phenotype.Mov Disord Clin Pract. 2024 Jan;11(1):76-85. doi: 10.1002/mdc3.13920. Epub 2023 Nov 28. Mov Disord Clin Pract. 2024. PMID: 38291835 Free PMC article. Review.
Cited by
-
Active site variants in STT3A cause a dominant type I congenital disorder of glycosylation with neuromusculoskeletal findings.Am J Hum Genet. 2021 Nov 4;108(11):2130-2144. doi: 10.1016/j.ajhg.2021.09.012. Epub 2021 Oct 14. Am J Hum Genet. 2021. PMID: 34653363 Free PMC article.
-
O-GlcNAcylation modulates expression and abundance of N-glycosylation machinery in an inherited glycosylation disorder.Cell Rep. 2024 Nov 26;43(11):114976. doi: 10.1016/j.celrep.2024.114976. Epub 2024 Nov 18. Cell Rep. 2024. PMID: 39561044 Free PMC article.
-
Genomic insights into the comorbidity between type 2 diabetes and schizophrenia.Schizophrenia (Heidelb). 2024 Feb 21;10(1):22. doi: 10.1038/s41537-024-00445-5. Schizophrenia (Heidelb). 2024. PMID: 38383672 Free PMC article.
-
Novel Copy Number Deletion Involving NUS1 Associated With Epilepsy, Tremor, and Intellectual Disability.Clin Case Rep. 2025 Jan 8;13(1):e70022. doi: 10.1002/ccr3.70022. eCollection 2025 Jan. Clin Case Rep. 2025. PMID: 39780902 Free PMC article.
-
Loss of NgBR causes neuronal damage through decreasing KAT7-mediated RFX1 acetylation and FGF1 expression.Cell Mol Life Sci. 2025 Apr 7;82(1):140. doi: 10.1007/s00018-025-05660-6. Cell Mol Life Sci. 2025. PMID: 40192836 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

