Pancreatic cancer induces muscle wasting by promoting the release of pancreatic adenocarcinoma upregulated factor
- PMID: 33731895
- PMCID: PMC8080719
- DOI: 10.1038/s12276-021-00582-2
Pancreatic cancer induces muscle wasting by promoting the release of pancreatic adenocarcinoma upregulated factor
Abstract
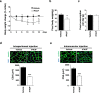
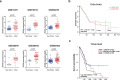
Cancer cachexia is a highly debilitating condition characterized by weight loss and muscle wasting that contributes significantly to the morbidity and mortality of pancreatic cancer. The factors that induce cachexia in pancreatic cancer are largely unknown. We previously showed that pancreatic adenocarcinoma upregulated factor (PAUF) secreted by pancreatic cancer cells is responsible for tumor growth and metastasis. Here, we analyzed the relation between pancreatic cancer-derived PAUF and cancer cachexia in mice and its clinical significance. Body weight loss and muscle weight loss were significantly higher in mice with Panc-1/PAUF tumors than in those with Panc-1/Mock tumors. Direct administration of rPAUF to muscle recapitulated tumor-induced atrophy, and a PAUF-neutralizing antibody abrogated tumor-induced muscle wasting in Panc-1/PAUF tumor-bearing mice. C2C12 myotubes treated with rPAUF exhibited rapid inactivation of Akt-Foxo3a signaling, resulting in Atrogin1/MAFbx upregulation, myosin heavy chain loss, and muscle atrophy. The neutrophil-to-lymphocyte ratio and body weight loss were significantly higher in pancreatic cancer patients with high PAUF expression than in those with low PAUF expression. Analysis of different pancreatic cancer datasets showed that PAUF expression was significantly higher in the pancreatic cancer group than in the nontumor group. Analysis of The Cancer Genome Atlas data found associations between high PAUF expression or a high DNA copy number and poor overall survival. Our data identified tumor-secreted circulating PAUF as a key factor of cachexia, causing muscle wasting in mice. Neutralizing PAUF may be a useful therapeutic strategy for the treatment of pancreatic cancer-induced cachexia.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

