How the O2-dependent Mg-protoporphyrin monomethyl ester cyclase forms the fifth ring of chlorophylls
- PMID: 33731920
- PMCID: PMC7610348
- DOI: 10.1038/s41477-021-00876-3
How the O2-dependent Mg-protoporphyrin monomethyl ester cyclase forms the fifth ring of chlorophylls
Abstract
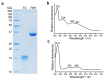
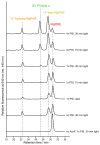
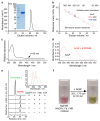
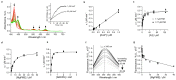
Mg-protoporphyrin IX monomethyl ester (MgPME) cyclase catalyses the formation of the isocyclic ring, producing protochlorophyllide a and contributing substantially to the absorption properties of chlorophylls and bacteriochlorophylls. The O2-dependent cyclase is found in both oxygenic phototrophs and some purple bacteria. We overproduced the simplest form of the cyclase, AcsF, from Rubrivivax gelatinosus, in Escherichia coli. In biochemical assays the di-iron cluster within AcsF is reduced by ferredoxin furnished by NADPH and ferredoxin:NADP+ reductase, or by direct coupling to Photosystem I photochemistry, linking cyclase to the photosynthetic electron transport chain. Kinetic analyses yielded a turnover number of 0.9 min-1, a Michaelis-Menten constant of 7.0 µM for MgPME and a dissociation constant for MgPME of 0.16 µM. Mass spectrometry identified 131-hydroxy-MgPME and 131-keto-MgPME as cyclase reaction intermediates, revealing the steps that form the isocyclic ring and completing the work originated by Sam Granick in 1950.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Porra RJ, Schäfer W, Gad’on N, Katheder I, Drews G, Scheer H. Origin of the two carbonyl oxygens of bacteriochlorophyll a Demonstration of two different pathways for the formation of ring E in Rhodobacter sphaeroides and Roseobacter denitrificans and a common hydratase mechanism for 3-acetyl group formation. Eur J Biochem. 1996;239:85–92. - PubMed
-
- Porra RJ, Urzinger M, Winkler J, Bubenzer C, Scheer H. Biosynthesis of the 3-acetyl and 131-oxo groups of bacteriochlorophyll a in the facultative aerobic bacterium, Rhodovulum sulfidophilum: The presence of both oxygenase and hydratase pathways for isocyclic ring formation. Eur J Biochem. 1998;257:185–191. - PubMed
-
- Wiesselmann M, Hebecker S, Borrero-de Acuña JM, Nimtz M, Bollivar D, Jänsch L, Moser J, Jahn D. Mg-protoporphyrin IX monomethyl ester cyclase from Rhodobacter capsulatus: Radical SAM-dependent synthesis of the isocyclic ring of bacteriochlorophylls. Biochem J. 2020 BCJ20200761. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

