Expansible residence decentralizes immune homeostasis
- PMID: 33731934
- PMCID: PMC8057530
- DOI: 10.1038/s41586-021-03351-3
Expansible residence decentralizes immune homeostasis
Abstract
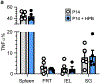
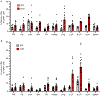
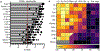
In metazoans, specific tasks are relegated to dedicated organs that are established early in development, occupy discrete locations and typically remain fixed in size. The adult immune system arises from a centralized haematopoietic niche that maintains self-renewing potential1,2, and-upon maturation-becomes distributed throughout the body to monitor environmental perturbations, regulate tissue homeostasis and mediate organism-wide defence. Here we examine how immunity is integrated within adult mouse tissues, and address issues of durability, expansibility and contributions to organ cellularity. Focusing on antiviral T cell immunity, we observed durable maintenance of resident memory T cells up to 450 days after infection. Once established, resident T cells did not require the T cell receptor for survival or retention of a poised, effector-like state. Although resident memory indefinitely dominated most mucosal organs, surgical separation of parabiotic mice revealed a tissue-resident provenance for blood-borne effector memory T cells, and circulating memory slowly made substantial contributions to tissue immunity in some organs. After serial immunizations or cohousing with pet-shop mice, we found that in most tissues, tissue pliancy (the capacity of tissues to vary their proportion of immune cells) enables the accretion of tissue-resident memory, without axiomatic erosion of pre-existing antiviral T cell immunity. Extending these findings, we demonstrate that tissue residence and organ pliancy are generalizable aspects that underlie homeostasis of innate and adaptive immunity. The immune system grows commensurate with microbial experience, reaching up to 25% of visceral organ cellularity. Regardless of the location, many populations of white blood cells adopted a tissue-residency program within nonlymphoid organs. Thus, residence-rather than renewal or recirculation-typifies nonlymphoid immune surveillance, and organs serve as pliant storage reservoirs that can accommodate continuous expansion of the cellular immune system throughout life. Although haematopoiesis restores some elements of the immune system, nonlymphoid organs sustain an accrual of durable tissue-autonomous cellular immunity that results in progressive decentralization of organismal immune homeostasis.
Figures





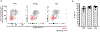





References
-
- Höfer T, Busch K, Klapproth K & Rodewald H-R Fate Mapping and Quantitation of Hematopoiesis In Vivo. Annu. Rev. Immunol 34, 449–478 (2016). - PubMed
-
- Janeway CA et al. Modes of cell:cell communication in the immune system. J. Immunol 135, 739s–742s (1985). - PubMed
-
- Qi H, Kastenmüller W & Germain RN Spatiotemporal basis of innate and adaptive immunity in secondary lymphoid tissue. Annu. Rev. Cell Dev. Biol 30, 141–67 (2014). - PubMed
-
- Bromley SK et al. The immunological synapse. Annu. Rev. Immunol 19, 375–96 (2001). - PubMed
Methods References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

