The Effect of Teratozoospermia on Sex Chromosomes in Human Embryos
- PMID: 33732009
- PMCID: PMC7959001
- DOI: 10.2147/TACG.S299349
The Effect of Teratozoospermia on Sex Chromosomes in Human Embryos
Abstract
Purpose: The aim of this study is to evaluate the effect of abnormal semen morphology on the frequency of sex chromosomal abnormalities in embryos obtained by ICSI, which represents the first to be studied in Egyptian population.
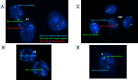
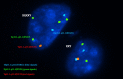
Methods: Forty-two couples suffering from male infertility due to teratozoospermia were divided into two groups: patients with severe and moderate teratozoospermia (group A and B, respectively). All involved couples were subjected to careful history taking and had a normal clinical examination and karyotype. Females were subjected to hormonal assays, pelvic ultrasound, hysterosalpingography and yielded normal results, while male partners were subjected to computerized semen analysis. Preimplantation genetic diagnosis was performed for all suitably developed embryos including embryo biopsy, fixation of biopsied cells and fluorescent in situ hybridization (FISH) analysis.
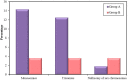
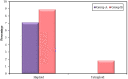
Results: Couples included in the two groups were found to be homogenous in terms of age of both partners and duration of infertility. Interpretation of FISH results was performed by evaluation of embryos' chromosomal constitution as regards abnormalities in chromosomes X, Y and 18. Twenty-seven embryos (48.2%) were found chromosomally abnormal in group A, while only 14 embryos (25.0%) were found chromosomally abnormal in group B. Aneuploidies involved only sex chromosomes were tripled in group A embryos when compared to their frequency in group B embryos (26.8% and 8.3%, respectively) with statistically significant difference between the two groups (p=0.002). Monosomies were the most common type of aneuploidy and were significantly higher in group A (14.3%) when compared to group B (3.6%) (p=0.047). Embryos with mosaic abnormalities were more common in group A (12.5%) when compared to group B (3.6%), however not statistically significantly different (p= 0.162). A significant difference between the two studied groups as regards the total number of potentially viable chromosomal abnormalities detected and the potentially viable sex chromosomal aneuploidies detected (p<0.001 and p=0.002), respectively.
Conclusion: The cases with severe teratozoospermia undergoing ICSI treatment can display a higher rate of sex chromosome aneuploidies in their embryos (threefold) than cases with moderate teratozoospermia.
Keywords: FISH; ICSI; aneuploidy; teratozoospermia.
© 2021 Mostafa Nayel et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

Similar articles
-
Assessment of numeric abnormalities of X, Y, 18, and 16 chromosomes in preimplantation human embryos before transfer.Am J Obstet Gynecol. 1995 Apr;172(4 Pt 1):1191-9; discussion 1199-201. doi: 10.1016/0002-9378(95)91479-x. Am J Obstet Gynecol. 1995. PMID: 7726256
-
Sequential comprehensive chromosome analysis on polar bodies, blastomeres and trophoblast: insights into female meiotic errors and chromosomal segregation in the preimplantation window of embryo development.Hum Reprod. 2013 Feb;28(2):509-18. doi: 10.1093/humrep/des394. Epub 2012 Nov 11. Hum Reprod. 2013. PMID: 23148203
-
Extended in vitro culture of human embryos demonstrates the complex nature of diagnosing chromosomal mosaicism from a single trophectoderm biopsy.Hum Reprod. 2019 Apr 1;34(4):758-769. doi: 10.1093/humrep/dez012. Hum Reprod. 2019. PMID: 30838420
-
Chromosome abnormalities in sperm from infertile men with normal somatic karyotypes: teratozoospermia.Cytogenet Genome Res. 2005;111(3-4):352-7. doi: 10.1159/000086910. Cytogenet Genome Res. 2005. PMID: 16192715 Review.
-
Sperm chromosomal abnormalities and their contribution to human embryo aneuploidy.Biol Reprod. 2019 Dec 24;101(6):1091-1101. doi: 10.1093/biolre/ioz125. Biol Reprod. 2019. PMID: 31318411 Review.
Cited by
-
Enhancing Intracytoplasmic Sperm Injection Outcomes With Zeta Sperm Selection: A Case Report.Cureus. 2024 Jul 18;16(7):e64809. doi: 10.7759/cureus.64809. eCollection 2024 Jul. Cureus. 2024. PMID: 39156240 Free PMC article.
-
A Comparative Cross-Platform Analysis to Identify Potential Biomarker Genes for Evaluation of Teratozoospermia and Azoospermia.Genes (Basel). 2022 Sep 25;13(10):1721. doi: 10.3390/genes13101721. Genes (Basel). 2022. PMID: 36292606 Free PMC article.
-
Delayed Consequences of the Toxic Effect of Paclitaxel on the Testes of Prepubertal Rats and Their Correction with p-Tyrosol.Bull Exp Biol Med. 2022 Jul;173(3):341-345. doi: 10.1007/s10517-022-05546-8. Epub 2022 Jul 19. Bull Exp Biol Med. 2022. PMID: 35852682
-
Pregnancy Outcomes in Double Stimulation versus Two Consecutive Mild Stimulations for IVF in Poor Ovarian Responders.J Clin Med. 2022 Nov 16;11(22):6780. doi: 10.3390/jcm11226780. J Clin Med. 2022. PMID: 36431256 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

