The Neurobiology of Selenium: Looking Back and to the Future
- PMID: 33732108
- PMCID: PMC7959785
- DOI: 10.3389/fnins.2021.652099
The Neurobiology of Selenium: Looking Back and to the Future
Abstract
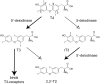
Eighteen years ago, unexpected epileptic seizures in Selenop-knockout mice pointed to a potentially novel, possibly underestimated, and previously difficult to study role of selenium (Se) in the mammalian brain. This mouse model was the key to open the field of molecular mechanisms, i.e., to delineate the roles of selenium and individual selenoproteins in the brain, and answer specific questions like: how does Se enter the brain; which processes and which cell types are dependent on selenoproteins; and, what are the individual roles of selenoproteins in the brain? Many of these questions have been answered and much progress is being made to fill remaining gaps. Mouse and human genetics have together boosted the field tremendously, in addition to traditional biochemistry and cell biology. As always, new questions have become apparent or more pressing with solving older questions. We will briefly summarize what we know about selenoproteins in the human brain, glance over to the mouse as a useful model, and then discuss new questions and directions the field might take in the next 18 years.
Keywords: GPX4; epilepsy; ferroptosis; genetics; neurodegeneration.
Copyright © 2021 Schweizer, Bohleber, Zhao and Fradejas-Villar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Roles of Selenoproteins in Brain Function and the Potential Mechanism of Selenium in Alzheimer's Disease.Front Neurosci. 2021 Mar 8;15:646518. doi: 10.3389/fnins.2021.646518. eCollection 2021. Front Neurosci. 2021. PMID: 33762907 Free PMC article. Review.
-
Seizures, ataxia and parvalbumin-expressing interneurons respond to selenium supply in Selenop-deficient mice.Redox Biol. 2022 Nov;57:102490. doi: 10.1016/j.redox.2022.102490. Epub 2022 Sep 24. Redox Biol. 2022. PMID: 36182809 Free PMC article.
-
Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis.Cell. 2018 Jan 25;172(3):409-422.e21. doi: 10.1016/j.cell.2017.11.048. Epub 2017 Dec 28. Cell. 2018. PMID: 29290465
-
Unveiling the molecular mechanisms behind selenium-related diseases through knockout mouse studies.Antioxid Redox Signal. 2010 Apr 1;12(7):851-65. doi: 10.1089/ars.2009.2912. Antioxid Redox Signal. 2010. PMID: 19803749 Review.
-
A Novel Organic Selenium Compound Exerts Unique Regulation of Selenium Speciation, Selenogenome, and Selenoproteins in Broiler Chicks.J Nutr. 2017 May;147(5):789-797. doi: 10.3945/jn.116.247338. Epub 2017 Mar 29. J Nutr. 2017. PMID: 28356430
Cited by
-
Selenoprotein P Concentrations in the Cerebrospinal Fluid and Serum of Individuals Affected by Amyotrophic Lateral Sclerosis, Mild Cognitive Impairment and Alzheimer's Dementia.Int J Mol Sci. 2022 Aug 30;23(17):9865. doi: 10.3390/ijms23179865. Int J Mol Sci. 2022. PMID: 36077261 Free PMC article.
-
Modulation of the Functional State of Mouse Neutrophils by Selenium Nanoparticles In Vivo.Int J Mol Sci. 2022 Nov 7;23(21):13651. doi: 10.3390/ijms232113651. Int J Mol Sci. 2022. PMID: 36362436 Free PMC article.
-
Selenium in Bodily Homeostasis: Hypothalamus, Hormones, and Highways of Communication.Int J Mol Sci. 2022 Dec 6;23(23):15445. doi: 10.3390/ijms232315445. Int J Mol Sci. 2022. PMID: 36499772 Free PMC article. Review.
-
Selenium Status in Paediatric Patients with Neurodevelopmental Diseases.Nutrients. 2022 Jun 8;14(12):2375. doi: 10.3390/nu14122375. Nutrients. 2022. PMID: 35745104 Free PMC article.
-
Correlation between selenium levels and selenoproteins expression in idiopathic generalized epilepsy: a study from Karachi.BMC Neurol. 2025 Jan 23;25(1):34. doi: 10.1186/s12883-024-03993-6. BMC Neurol. 2025. PMID: 39849427 Free PMC article.
References
-
- Aygun C., Celik F. C., Nural M. S., Azak E., Kucukoduk S., Ogur G., et al. (2012). Simplified gyral pattern with cerebellar hypoplasia in Sedaghatian type spondylometaphyseal dysplasia: a clinical report and review of the literature. Am. J. Med. Genet. A 158A 1400–1405. 10.1002/ajmg.a.35306 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

