Elamipretide (SS-31) Improves Functional Connectivity in Hippocampus and Other Related Regions Following Prolonged Neuroinflammation Induced by Lipopolysaccharide in Aged Rats
- PMID: 33732135
- PMCID: PMC7956963
- DOI: 10.3389/fnagi.2021.600484
Elamipretide (SS-31) Improves Functional Connectivity in Hippocampus and Other Related Regions Following Prolonged Neuroinflammation Induced by Lipopolysaccharide in Aged Rats
Abstract
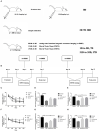
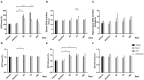
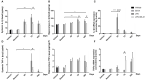
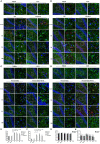
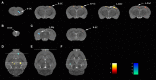
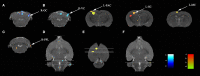
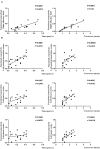
Neuroinflammation has been recognized as a major cause for neurocognitive diseases. Although the hippocampus has been considered an important region for cognitive dysfunction, the influence of hippocampal neuroinflammation on brain functional connectivity (FC) has been rarely studied. In this study, lipopolysaccharide (LPS) was used to induce systemic inflammation and neuroinflammation in the aged rat brain, while elamipretide (SS-31) was used for treatment. Systemic and hippocampal inflammation were determined using ELISA, while astrocyte responses during hippocampal neuroinflammation were determined by interleukin 1 beta (IL-1β)/tumor necrosis factor alpha (TNFα) double staining immunofluorescence. Oxidative stress was determined by reactive oxidative species (ROS), electron transport chain (ETC) complex, and superoxide dismutase (SOD). Short- (<7 days) and long-term (>30 days) learning and spatial working memory were tested by the Morris water maze (MWM). Resting-state functional magnetic resonance imaging (rs-fMRI) was used to analyze the brain FC by placing seed voxels on the left and right hippocampus. Compared with the vehicle group, rats with the LPS exposure showed an impaired MWM performance, higher oxidative stress, higher levels of inflammatory cytokines, and astrocyte activation in the hippocampus. The neuroimaging examination showed decreased FC on the right orbital cortex, right olfactory bulb, and left hippocampus on day 3, 7, and 31, respectively, after treatment. In contrast, rats with SS-31 treatment showed lower levels of inflammatory cytokines, less astrocyte activation in the hippocampus, and improved MWM performance. Neuroimaging examination showed increased FC on the left-parietal association cortex (L-PAC), left sensory cortex, and left motor cortex on day 7 with the right flocculonodular lobe on day 31 as compared with those without SS-31 treatment. Our study demonstrated that inhibiting neuroinflammation in the hippocampus not only reduces inflammatory responses in the hippocampus but also improves the brain FC in regions related to the hippocampus. Furthermore, early anti-inflammatory treatment with SS-31 has a long-lasting effect on reducing the impact of LPS-induced neuroinflammation.
Keywords: cognition; elamipretide; functional connectivity; lipopolysaccharide; neuroinflammation.
Copyright © 2021 Liu, Fu, Wu, Nie, Liu, Wang, Xiao, Yang, Kan and Fan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Altered functional connectivity and topology structures in default mode network induced by inflammatory exposure in aged rat: A resting-state functional magnetic resonance imaging study.Front Aging Neurosci. 2022 Nov 17;14:1013478. doi: 10.3389/fnagi.2022.1013478. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36466609 Free PMC article.
-
Elamipretide (SS-31) improves mitochondrial dysfunction, synaptic and memory impairment induced by lipopolysaccharide in mice.J Neuroinflammation. 2019 Nov 20;16(1):230. doi: 10.1186/s12974-019-1627-9. J Neuroinflammation. 2019. PMID: 31747905 Free PMC article.
-
Insulin treatment protects the brain against neuroinflammation by reducing cerebral cytokines and modulating mitochondrial function.Brain Res Bull. 2019 Jul;149:120-128. doi: 10.1016/j.brainresbull.2019.04.011. Epub 2019 Apr 16. Brain Res Bull. 2019. PMID: 31002914
-
Curcumin attenuates neuroinflammation and improves cognitive function in a rat model of febrile convulsions.Sci Rep. 2025 Apr 28;15(1):14841. doi: 10.1038/s41598-025-99486-8. Sci Rep. 2025. PMID: 40295644 Free PMC article.
-
A standardised Andrographis paniculata Burm. Nees aqueous extract prevents Lipopolysaccharide-induced cognitive deficits through suppression of inflammatory cytokines and oxidative stress mediators.J Adv Res. 2018 Nov 30;16:87-97. doi: 10.1016/j.jare.2018.11.005. eCollection 2019 Mar. J Adv Res. 2018. PMID: 30899592 Free PMC article.
Cited by
-
Transcranial near-infrared laser improves postoperative neurocognitive disorder in aged mice via SIRT3/AMPK/Nrf2 pathway.Front Neurosci. 2023 Jan 24;16:1100915. doi: 10.3389/fnins.2022.1100915. eCollection 2022. Front Neurosci. 2023. PMID: 36760797 Free PMC article.
-
Mitochondrial DNA leakage: underlying mechanisms and therapeutic implications in neurological disorders.J Neuroinflammation. 2025 Feb 7;22(1):34. doi: 10.1186/s12974-025-03363-0. J Neuroinflammation. 2025. PMID: 39920753 Free PMC article. Review.
-
Disentangling Mitochondria in Alzheimer's Disease.Int J Mol Sci. 2021 Oct 26;22(21):11520. doi: 10.3390/ijms222111520. Int J Mol Sci. 2021. PMID: 34768950 Free PMC article. Review.
-
Altered functional connectivity and topology structures in default mode network induced by inflammatory exposure in aged rat: A resting-state functional magnetic resonance imaging study.Front Aging Neurosci. 2022 Nov 17;14:1013478. doi: 10.3389/fnagi.2022.1013478. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36466609 Free PMC article.
-
Elamipretide alleviates pyroptosis in traumatically injured spinal cord by inhibiting cPLA2-induced lysosomal membrane permeabilization.J Neuroinflammation. 2023 Jan 7;20(1):6. doi: 10.1186/s12974-023-02690-4. J Neuroinflammation. 2023. PMID: 36609266 Free PMC article.
References
-
- Alfonso-Loeches S., Urena-Peralta J. R., Morillo-Bargues M. J., Oliver-De La Cruz J., Guerri C. (2014). Role of mitochondria ROS generation in ethanol-induced NLRP3 inflammasome activation and cell death in astroglial cells. Front. Cell. Neurosci. 8:216. 10.3389/fncel.2014.00216 - DOI - PMC - PubMed
-
- Bauernfeind F. G., Horvath G., Stutz A., Alnemri E. S., MacDonald K., Speert D., et al. . (2009). Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 78−791. 10.4049/jimmunol.0901363 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

