A Journey From SARS-CoV-2 to COVID-19 and Beyond: A Comprehensive Insight of Epidemiology, Diagnosis, Pathogenesis, and Overview of the Progress into Its Therapeutic Management
- PMID: 33732150
- PMCID: PMC7957225
- DOI: 10.3389/fphar.2021.576448
A Journey From SARS-CoV-2 to COVID-19 and Beyond: A Comprehensive Insight of Epidemiology, Diagnosis, Pathogenesis, and Overview of the Progress into Its Therapeutic Management
Abstract
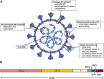
The 2019 novel coronavirus (2019-nCoV), commonly known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or coronavirus disease 2019 (COVID-19), was first revealed in late 2019 in Wuhan city, Hubei province, China. It was subsequently spread globally and thereby declared as a pandemic by WHO in March 2020. The disease causes severe acute respiratory illness and is highly contagious due to the fast-onward transmission. As of the mid of November 2020, the disease has affected 220 countries with more than 16 million active cases and 1.3 million deaths worldwide. Males, pregnant women, the elderly, immunosuppressed patients, and those with underlying medical conditions are more vulnerable to the disease than the general healthy population. Unfortunately, no definite treatment is available. Although remdesivir as an antiviral had been approved for use in those above 12 years of age and 40 kg weight group, it has been observed to be ineffective in large-scale SOLIDARITY trials by WHO. Moreover, dexamethasone has been found to increase the recovery rate of ventilated patients; oxygen and inhaled nitric oxide as a vasodilator have been given emergency expanded access. In addition, more than 57 clinical trials are being conducted for the development of the vaccines on various platforms. Two vaccines were found to be significantly promising in phase III results. It is concluded that till the approval of a specific treatment or development of a vaccine against this deadly disease, the preventive measures should be followed strictly to reduce the spread of the disease.
Keywords: COVID-19; SARS-CoV-2; azithromycin; coronavirus; dexamethasone; hydroxychloroquine; remdesivir; vaccines.
Copyright © 2021 Shoaib, Ahmed, Sikandar, Yousuf and Saleem.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Abbaspour Kasgari H., Moradi S., Shabani A. M., Babamahmoodi F., Davoudi Badabi A. R., Davoudi L., et al. (2020). Evaluation of the efficacy of sofosbuvir plus daclatasvir in combination with ribavirin for hospitalized COVID-19 patients with moderate disease compared with standard care: a single-centre, randomized controlled trial. J. Antimicrob. Chemother. 75, 3373–3378. 10.1093/jac/dkaa33 - DOI - PMC - PubMed
-
- Ahn M. (2020). How South Korea flattened the coronavirus curve with technology. Global Health NewsWire; Available at: https://globalhealthnewswire.com/technology/2020/04/22/how-south-korea-f... (Accessed June 17, 2020).
-
- Akram J., Azhar S., Shahzad M., Latif W., Khan K. S. (2020). Pakistan randomized and observational trial to evaluate coronavirus treatment (PROTECT) of hydroxychloroquine, oseltamivir and azithromycin to treat newly diagnosed patients with COVID-19 infection who have no comorbidities like diabetes mellitus: a structured summary of a study protocol for a randomized controlled trial. Trials 21, 702. 10.1186/s13063-020-04616-4 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

